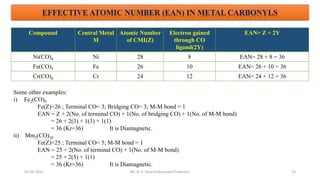
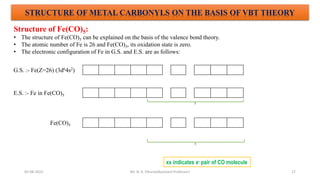
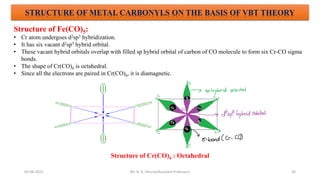
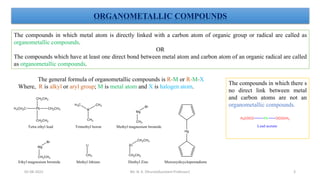
The document discusses organometallic compounds, specifically metal carbonyls. It provides classifications of organometallic compounds and metal carbonyls. It then describes the properties of two important metal carbonyls: nickel tetracarbonyl [Ni(CO)4] and iron pentacarbonyl [Fe(CO)5]. Nickel tetracarbonyl is a colorless liquid that decomposes upon heating to give nickel and carbon monoxide. Iron pentacarbonyl is a yellow toxic liquid that decomposes upon heating to give iron and carbon monoxide or dimerizes upon exposure to light. Both compounds react with acids to form metal salts and carbon monoxide.


![NOMENCLATURE OF ORGANOMETALLIC COMPOUNDS
1. Nomenclature of simple compounds:
The simple alkyl or aryl organometallic compounds of metal are named by writing the name of alkyl or aryl
group formed by the name of metal.
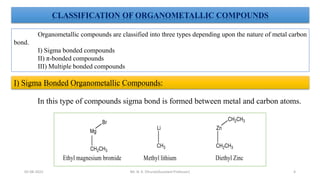
e.g.: CH3-Li Methyl lithium C2H5MgBr Ethyl magnesium bromide
(C2H5)2Zn Diethyl zinc C6H5MgCl Phenyl magnesium chloride
2. Nomenclature of carbonyls:
a) The compounds containing CO ligands are called metal carbonyls.
In neutral metal carbonyls, oxidation state of metal is zero and therefore need not be mentioned.
e.g., Ni(CO)4 Tetracarbonyl nickel Cr(CO)6 Hexacarbonyl chromium
Mn2(CO)10 Decacarbonyl dimanganese Co2(CO)8 Octacarbonyl dicobalt
Fe2(CO)9 Nonacarbonyl diiron [V(CO)6]- Hexacarbonyl vanadate(-I)
b) For bridging ligands use the Greek letter ‘’ before its name. If more than one bridging ligand are present, indicate
their number by using prefixes di-, tri-, tetra- etc.
e.g., [(CO)3Co(CO)2Co(CO)3 Di--carbonyl bis-(tricarbonylcobalt)
[(CO)3Fe(CO)3Fe(CO)3] Tri--carbonyl bis-(tricarbonyliron)
6
05-08-2022 Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-6-320.jpg)
![NOMENCLATURE OF ORGANOMETALLIC COMPOUNDS
2. Nomenclature of carbonyls:
c) If the metal carbonyls contains metal-metal bonds, then such carbonyls are classified as symmetrical or
unsymmetrical.
For the symmetrical metal carbonyls, the name are given by using the prefixes bis, tris, etc.
e.g., [(CO)4Co-Co(CO)4] Bis-(tetracarbonyl cobalt)
For the unsymmetrical metal carbonyls, one central metal atom and its ligands are treated as a ligand on the other
central metal atom.
e.g., [(CO)4Co-Re-(CO)5] Pentacarbonyl(tetracarbonyl cobaltio) rhenium
3. Nomenclature of σ and π bonded ligands:
a) To distinguish between one carbon bonded and multiple carbon bonded ligands the notation σ and π used.
e.g., Cyclopentadiene(C5H5)
(C5H5) Li σ-C5H5
(C5H5)2Fe π-C5H5
7
05-08-2022 Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-7-320.jpg)
![3. Nomenclature of σ and π bonded ligands:
b) In case of unsaturated ligands, the prefix ‘η’ is used. For one carbon bonded ligand use monohapto (η1), two
carbon bonded use dihapto (η2) and so on.
According to latest IUPAC convention, η notation is recommended.
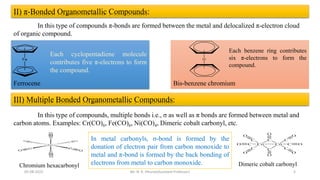
e.g., Fe(C5H5)2 Bis(η5-cyclopentadienyl)iron [Ferrocene]
Cr(C6H6)2 Bis(η6-benzene)chromium
Co(CO)3(π-C3H5) (η3-allyl)tricarbonyl cobalt
(C6H6)Cr(CO)3 (η6-benzene)tricarbonyl chromium
Fe2(CO)4(C5H5)2 Bis(η5-cyclopentadienyl)tetracarbonyl diiron
Fe(CO)2(σ-C5H5)(π-C5H5) (η1-cyclopentadienyl) (η5-cyclopentadienyl)dicarbonyl iron
Fe(CO)3(C4H6) (η4-butadiene)tricarbonyl iron
Mn(CO)5(-CH2-CH=CH2) (η3-allyl)pentacarbonyl manganese
NOMENCLATURE OF ORGANOMETALLIC COMPOUNDS
8
05-08-2022 Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-8-320.jpg)

![METAL CARBONYLS
Metal carbonyls are organometallic compounds of transition metals and carbon monoxide ligand (π acceptor
ligand or π acid) .
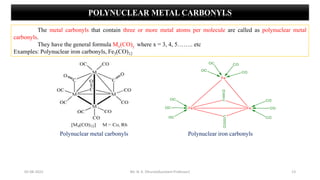
They have the general formula Mx(CO)y.
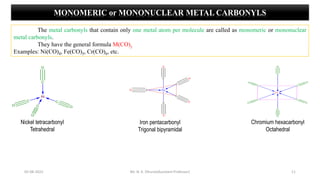
e.g., Cr(CO)6, Fe(CO)5, Ni(CO)4, etc.
Metal carbonyls possess following interesting properties:
1) They are gases, liquids or solids of low melting point. This property indicates that the bonding in them is covalent.
2) In these compounds oxidation state of metal is zero {except [V(CO)6]-1}.
3) All the metal carbonyls are diamagnetic in nature {except [V(CO)6]-1 which is paramagnetic}
10
05-08-2022
Classification of Metal Carbonyls
Metal carbonyls are classified into three types:
1) Monomeric or mononuclear metal carbonyls
2) Bridge Metal Carbonyls
3) Polynuclear Metal Carbonyls
Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-10-320.jpg)

![05-08-2022 14
I) Nickel Carbonyl : [Ni(CO)4]{Nickel tetracarbonyl}
Method of Preparation:
i) Direct synthesis by the action of carbon monoxide on metal:[Monds Process, 1890]
It is prepared by passing carbon monoxide over freshly reduced nickel at 303-323K at one atmospheric
pressure.
Ni + 4CO Ni(CO)4
303-323K (40
o
C)
1atm pressure
ii) Action of carbon monoxide on nickel salt (NaI2) in the presence of copper.
NiI2 + 4CO + 2Cu Ni(CO)4 + Cu2I2
NiS + 4CO + 2Cu Ni(CO)4 + Cu2S
Physical Properties:
i) It is colorless liquid.
ii) B.P. is 43
o
C(316K).
iii) Freezing point is -3
o
C(270K)
iv) It is soluble in organic solvents like benzene, ether, etc.
v) It is insoluble in water.
vi) It is highly toxic and inflammable in nature.
Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-14-320.jpg)
![05-08-2022 15
I) Nickel Carbonyl : [Ni(CO)4]{Nickel tetracarbonyl}
Chemical Properties:
i) Action of Heat: It decomposes on heating to give nickel and carbon monoxide.
Ni(CO)4 Ni + 4CO
ii) Action of Sulfuric acid: It reacts with sulfuric acid to give nickel sulfate.
Ni(CO)4 + H2SO4 NiSO4 + 4CO + H2
It slowly react with HCl and does not react with HBr or HI.
iii) Action of Halogen: It react with chloride to give nickel chloride and nickel carbonyl chloride.
Ni(CO)4 + Cl2 NiCl2 + 4CO
2Ni(CO)4 + Cl2 Ni(CO)6Cl2 + 2CO
It reacts with bromine to give nickel bromide.
Ni(CO)4 + Br2 NiBr2 + 4CO
Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-15-320.jpg)
![05-08-2022 16
I) Nickel Carbonyl : [Ni(CO)4]{Nickel tetracarbonyl}
iv) Action of ligands: It reacts with ligand like pyridine or o-phenanthroline to give disubstituted products in which two
CO molecules are replaced by ligand.
Ni(CO)4 + 2Py Ni(CO)2(Py)2 + 2CO
Ni(CO)4 + (Phen) Ni(CO)2(Phen) + 2CO
Ni + 4CO
NiSO4 + 4CO + H2
Ni(CO)4
NiX2 + 4CO
Ni(CO)2(Py)2 / Ni(CO)2(Phen)
H2SO4
X2 (X= Cl, Br)
Py/Phen
-2CO
Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-16-320.jpg)
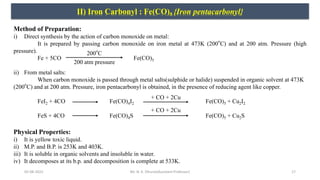
![05-08-2022 18
II) Iron Carbonyl : Fe(CO)5 {Iron pentacarbonyl}
Chemical Properties:
i) Action of heat: When iron pentacarbonyl is heated at 553K, it decomposes to give iron and carbon monoxide.
Fe(CO)5 Fe + 5CO
ii) Action of light: When iron pentacarbonyls is exposed to sunlight or irradiated with UV light, dimerization takes
place to give Nonacarbonyl diiron[Fe2(CO)9]
2Fe(CO)5 Fe2(CO)9 + CO
iii) Action of acid: When iron pentacarbonyl is heated with dilute acid gives salt.
Fe(CO)5+ 2HCl FeCl2 + 5CO + H2
iv) Action of alkali: On reaction with NaOH, iron pentacarbonyl gives yellow compound i.e., sodium carbonyl hydride.
Fe(CO)5+ 3NaOH Na[Fe(CO)4H] + Na2CO3 + H2O
v) Action with ammonia: Iron pentacarbonyl reacts with ammonia ( & water) to give carbamic acid.
Fe(CO)5+ NH3 + H2O [Fe(CO)4H2] + NH2COOH
Sunlight
UV
Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-18-320.jpg)
![05-08-2022 19
II) Iron Carbonyl : Fe(CO)5 {Iron pentacarbonyl}
vi) Action with halogen: Iron pentacarbonyls react with halogen to form iron carbonyl halide.
Fe(CO)5 + X2 [Fe(CO)4X2] + CO [X= Cl, Br, I]
vii) Reducing action: Iron pentacarbonyl acts as a reducing agent. It reduces the following:
a) It reduces carbon tetrachloride to dicarbon hexachloride.
Fe(CO)5 + 2CCl4 C2Cl6 + FeCl2 + 5CO
b) It reduces sulphuryl chloride to Sulphur dioxide.
Fe(CO)5 + SO2Cl2 FeCl2 + 5CO + SO2
c) It reduces SnCl4 to SnCl2. Fe(CO)4Cl2 further reacts with SnCl2 to form addition compound.
Fe(CO)5 + SnCl4 Fe(CO)4Cl2 + SnCl2 + CO
d) It reduces SbCl5 to SbCl3. Fe(CO)4Cl2 further reacts with SbCl3 to form addition compound.
Fe(CO)5 + SbCl5 Fe(CO)4Cl2 + SbCl3
Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-19-320.jpg)
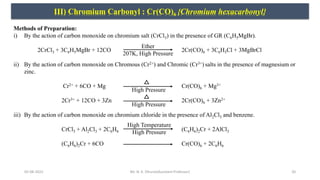
![05-08-2022 21
III) Chromium Carbonyl : Cr(CO)6 {Chromium hexacarbonyl}
Physical Properties:
i) It forms colorless rhombic crystal.
ii) M.P. is 422K.
iii) It sublime on heating.
iv) It is diamagnetic in nature.
v) It is soluble in organic solvent and insoluble in water.
Chemical Properties:
i) Reaction with halogen: It decomposes by fluorine and chlorine (except Br2 & I2) to form respective salts.
2Cr(CO)6 + 5F2 2CrF5 + 12CO
2Cr(CO)6 + 5Cl2 2CrCl5 + 12CO
ii) Reaction with sodium: It reacts with sodium to form chromium carbonylate.
2Na + Cr(CO)6 [2Na+][Cr(CO)6]
2-
+ CO
iii) Reaction with ligand: When chromium carbonyl reacts with ligand, gives substitution product ( by replacing 3CO
molecules).
Cr(CO)6 + 3Py Cr(CO)3(Py)3 + 3CO
iv) Reaction with unsaturated hydrocarbons: It combines with unsaturated hydrocarbon like cyclopentadiene at high
temperature and pressure to give a π-complex.
2Cr(CO)6 + 2C5H5 (C5H5)2Cr(CO)6
Mr. N. K. Dhurve(Assistant Professor)](https://image.slidesharecdn.com/unit-ii-inorganicchemistry9sem-vi-220805180101-d4eef061/85/UNIT-II-INORGANIC-CHEMISTRY9SEM-VI-pptx-21-320.jpg)