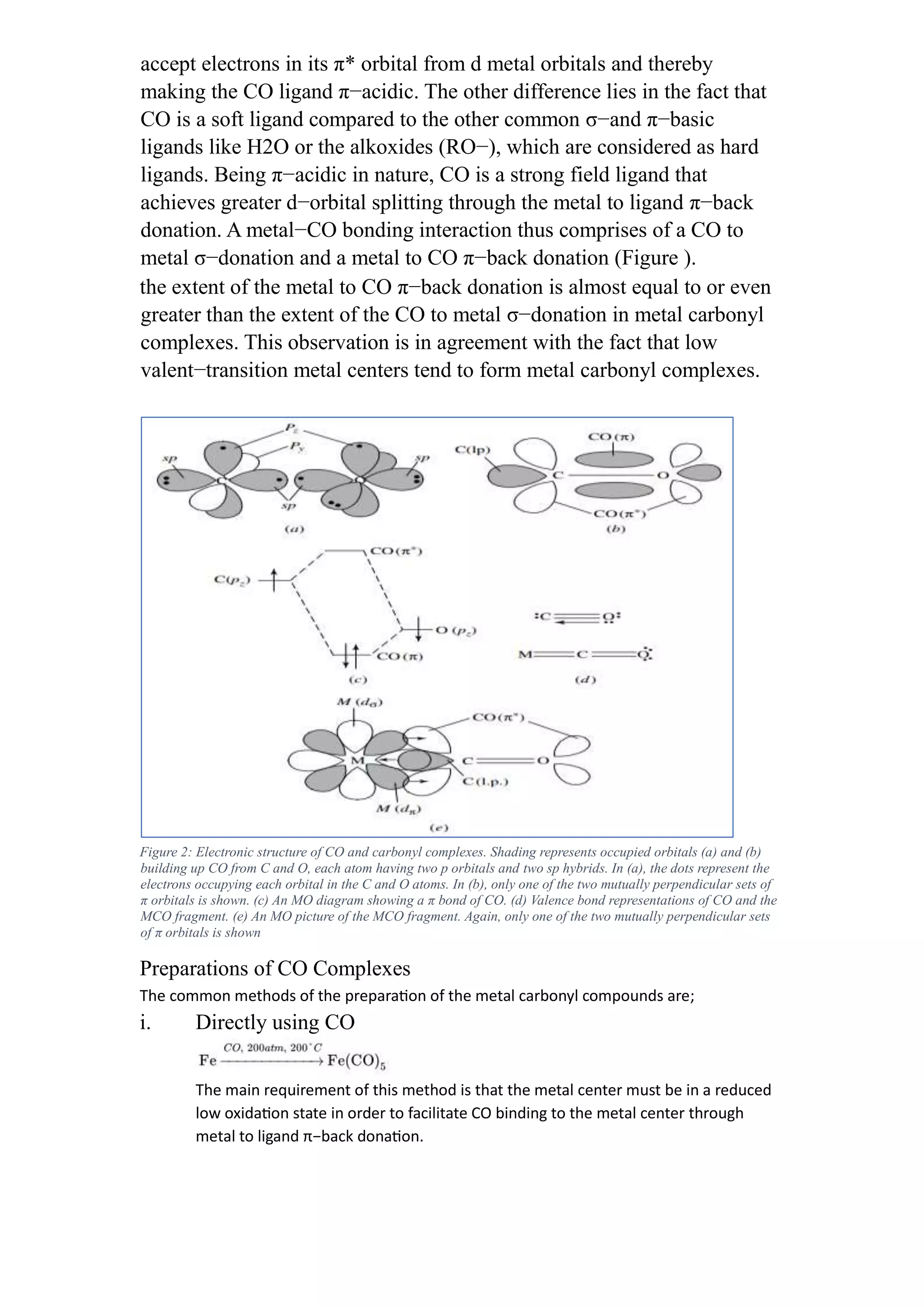
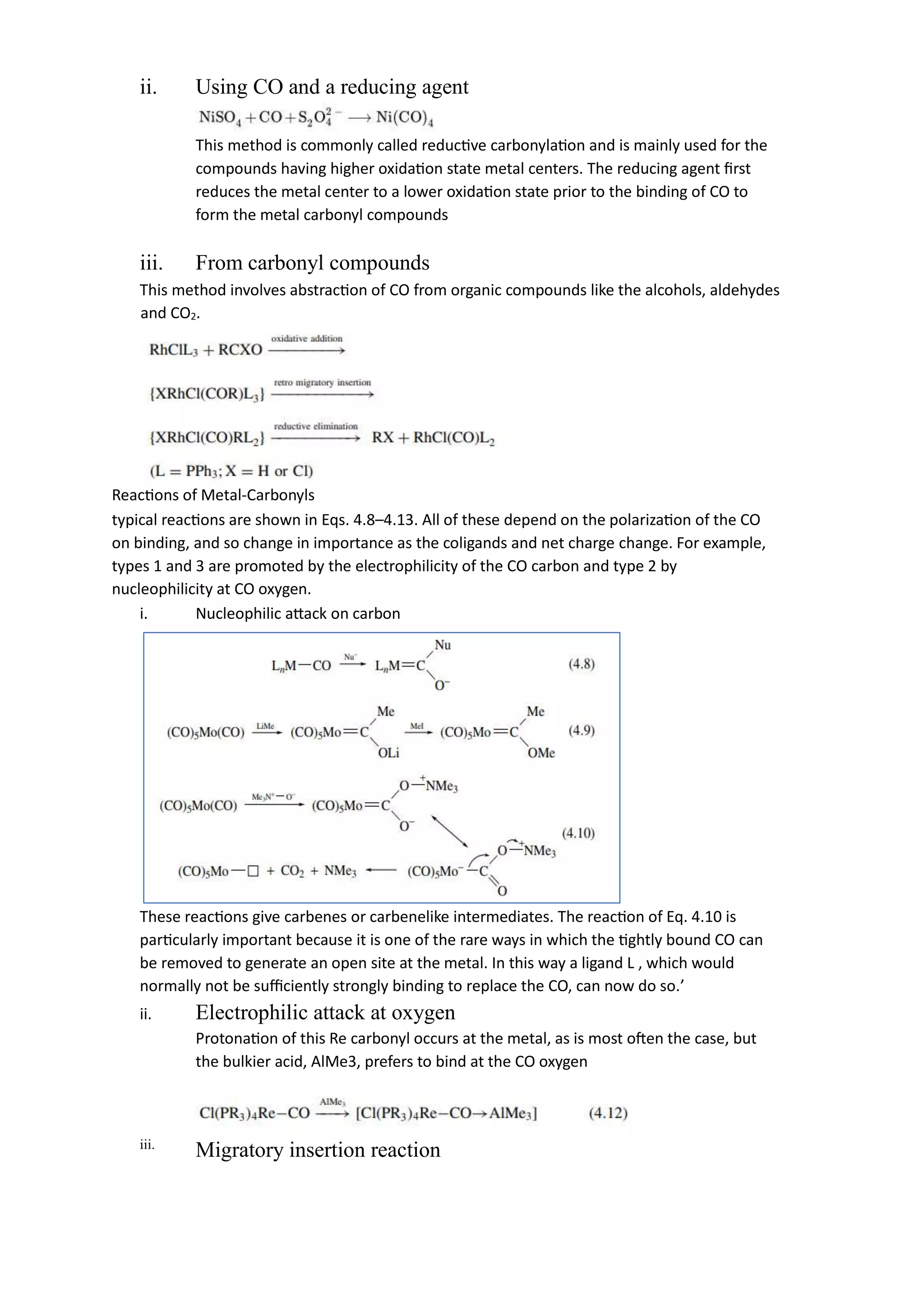
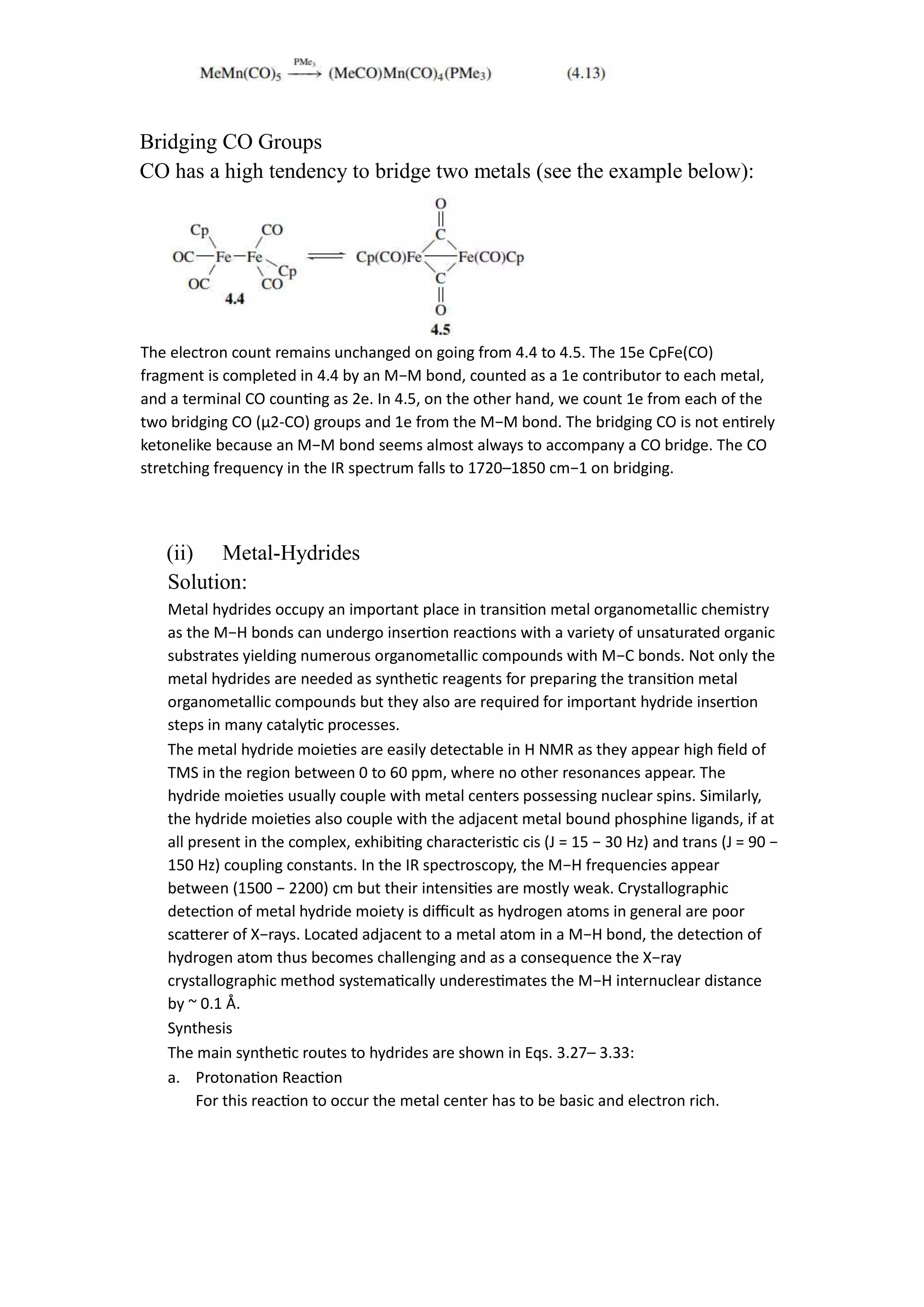
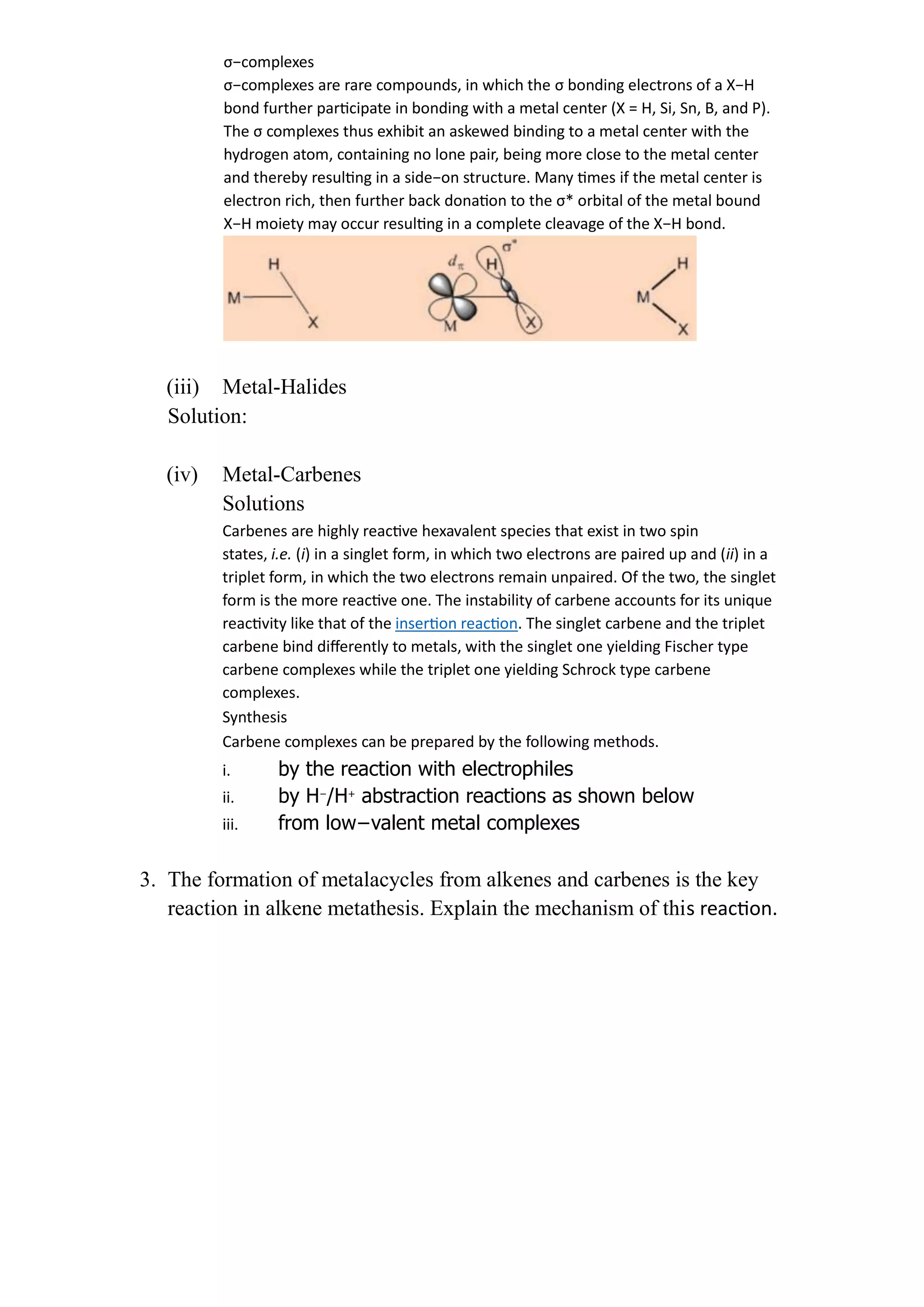
This document discusses metal-ligand bonding, including:
1) Drawing and labeling the molecular orbital diagram for a M-L6 complex, specifically [Co(NH3)6]3+.
2) Discussing metal-carbonyl, metal-hydride, metal-halide, and metal-carbene bonding, including types, synthesis, and reactions of each.
3) Explaining the mechanism of the formation of metalacycles from alkenes and carbenes, which is the key reaction in alkene metathesis.
![Assignment 1 Metal-Ligand Bonds
1. a) Draw and label the general molecular orbital diagram for a M-L6
complex ion, [Co(NH3)6]3+
. Identify all the orbitals used for bonding,
nonbonding and antibonding.
Solution:
Figure 1:MO diagram for M-L6 ([Co(NH3)6]3+
complex
i. Bonding Orbitals: a1g, eg(dx2
-y2
, dz2
), t1u
ii. Non-bonding Orbitals: T2g (dxy, dyz,dxz)
iii. Anti-bonding orbitals: eg
*
, A1g
*
, T1u
*
2. Discuss the following M-L bonding under subtopics: Types, Synthesis
and reactions.
(i) Metal-Carbonyls.
Solution:
The carbonyl ligand (CO) distinguishes itself from other ligands in many
respects. For example, unlike the alkyl ligands, the carbonyl (CO) ligand
is unsaturated thus allowing not only the ligand to σ−donate but also to](https://image.slidesharecdn.com/assignment1-230920160015-7523ba3b/75/Assignment1-docx-1-2048.jpg)