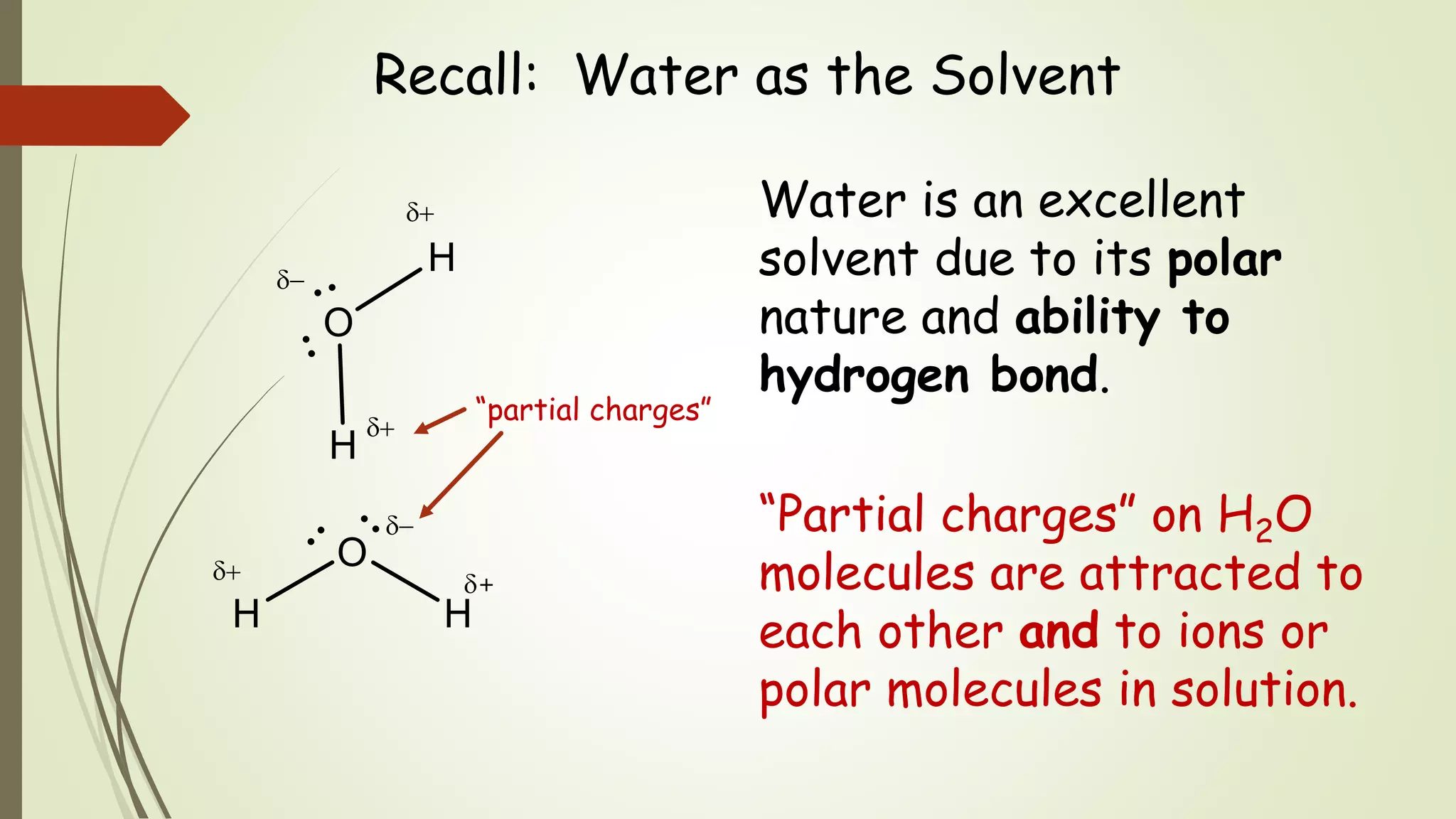
A solution is a homogeneous mixture of two or more substances. It consists of a solute dissolved in a solvent. Solutions can be mixtures of solids, liquids, or gases. Water is an excellent solvent due to its polar nature and ability to hydrogen bond. The formation of a solution is a physical process based on the tendency toward mixing and a match between the intermolecular forces of the solute and solvent. If these forces are similar, mixing will occur spontaneously to form a solution.