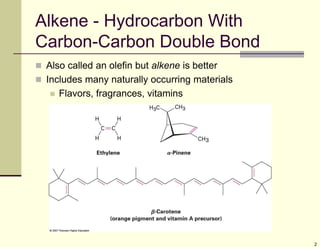
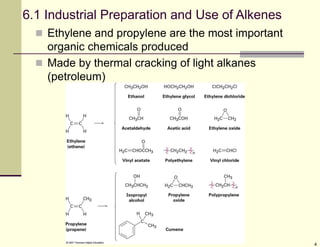
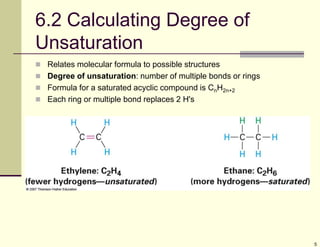
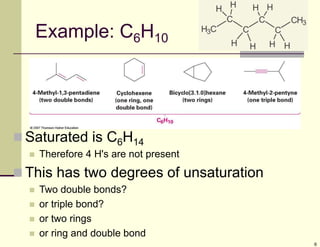
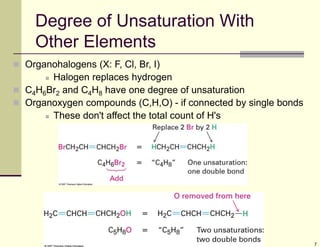
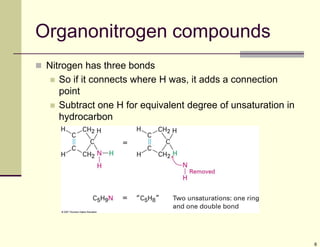
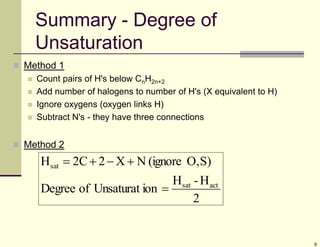
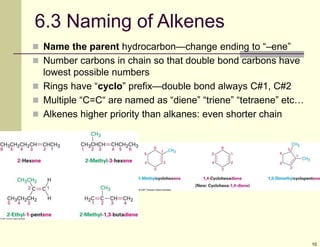
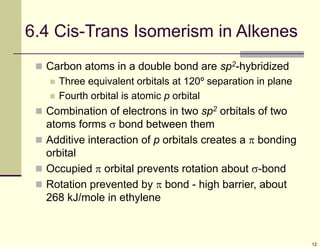
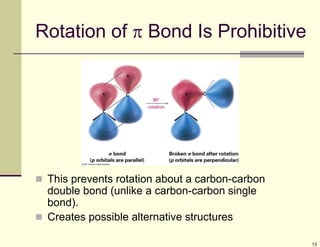
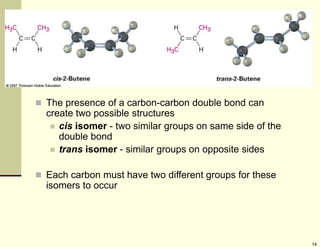
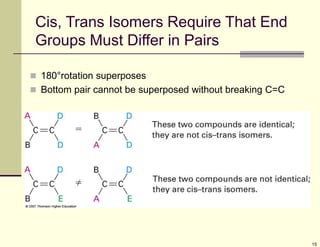
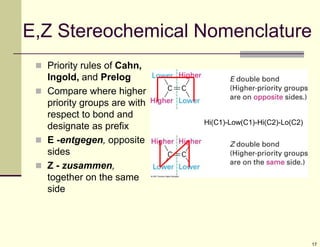
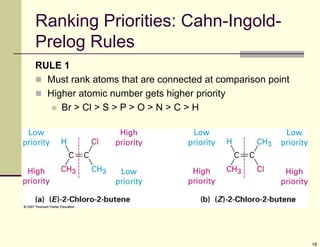
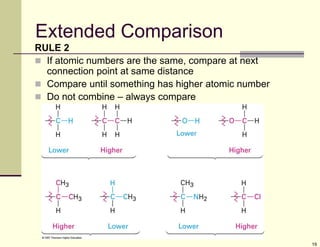
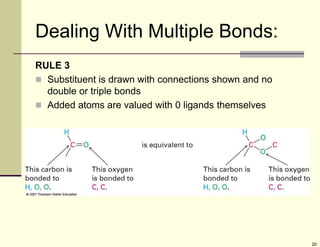
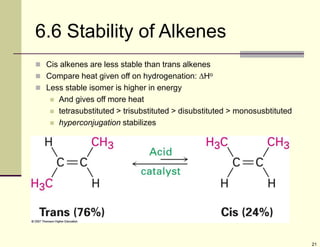
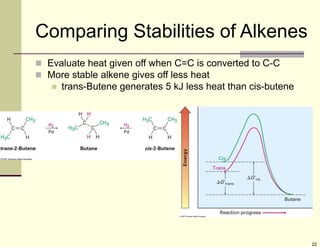
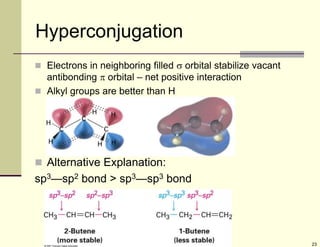
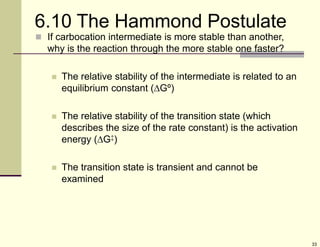
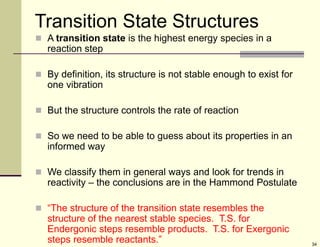
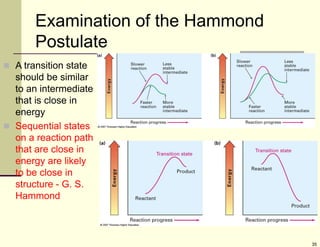
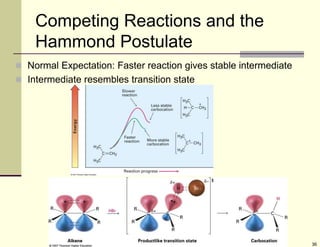
1) Alkenes are hydrocarbons containing a carbon-carbon double bond. They include many naturally occurring compounds like flavors and fragrances.
2) This chapter focuses on the general reaction of alkenes, which is electrophilic addition. It examines the consequences of alkene stereoisomerism and how double bonds are present in most organic molecules.
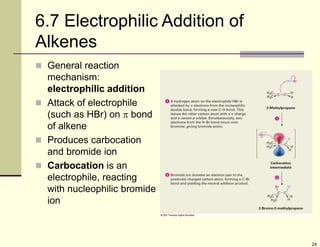
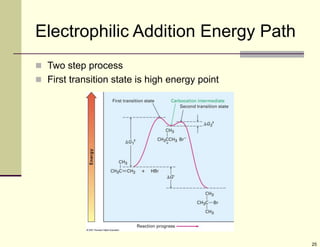
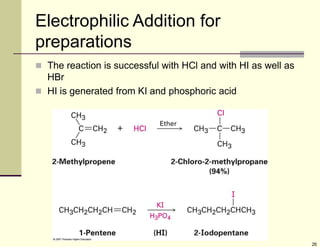
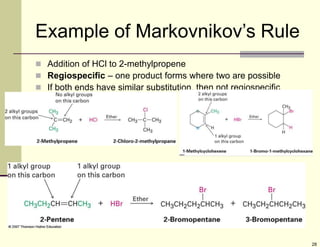
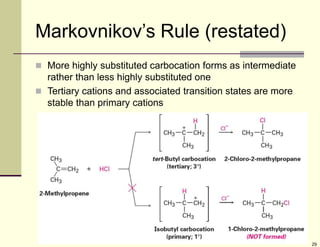
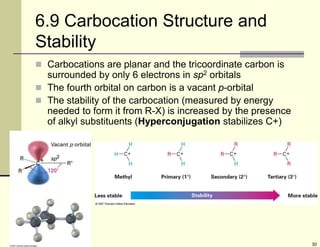
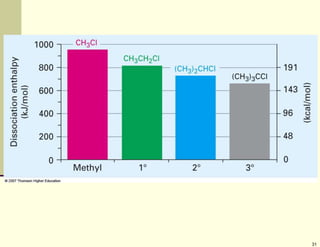
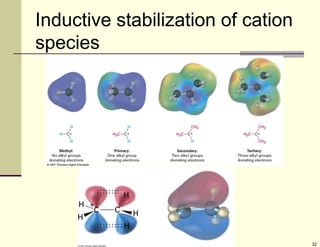
3) Electrophilic addition of alkenes involves the attack of an electrophile like HBr on the pi bond, forming a carbocation intermediate that then reacts with the bromide ion. This two-step process allows preparations using HCl or HI as well.