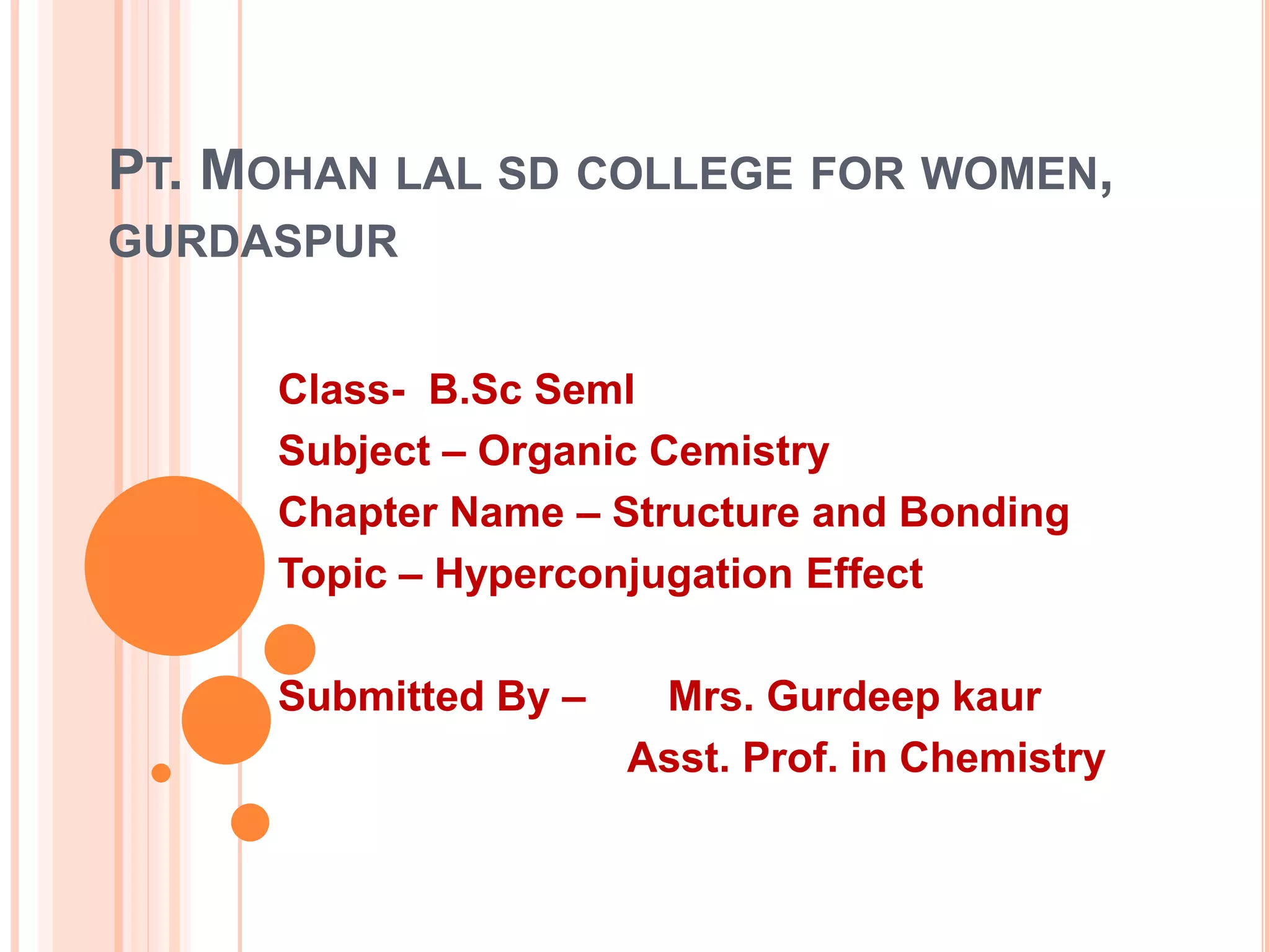
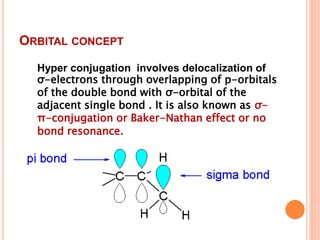
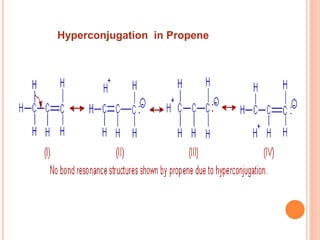
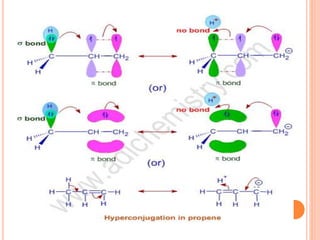
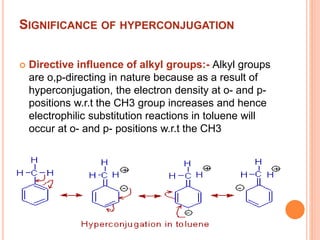
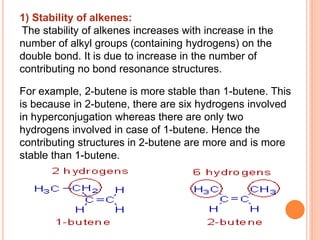
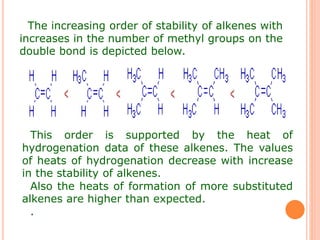
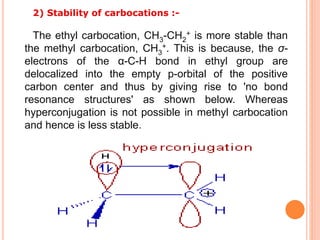
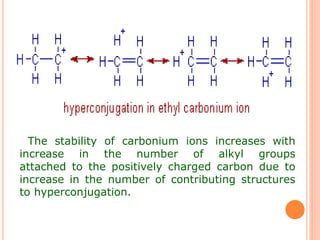
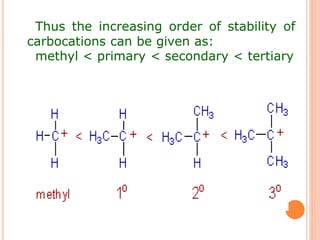
This document discusses the concept of hyperconjugation, which involves the delocalization of sigma electrons from an adjacent C-H bond into an empty p-orbital of an unsaturated system like an alkene or benzene ring. This effect increases the stability of alkenes and carbocations with more alkyl substituents by allowing for additional no bond resonance structures. The stability of alkenes and carbocations increases with the number of alkyl groups due to greater hyperconjugative stabilization from more C-H bonds. Hyperconjugation is an important effect that helps explain the observed stability and reactivity patterns of unsaturated organic compounds.