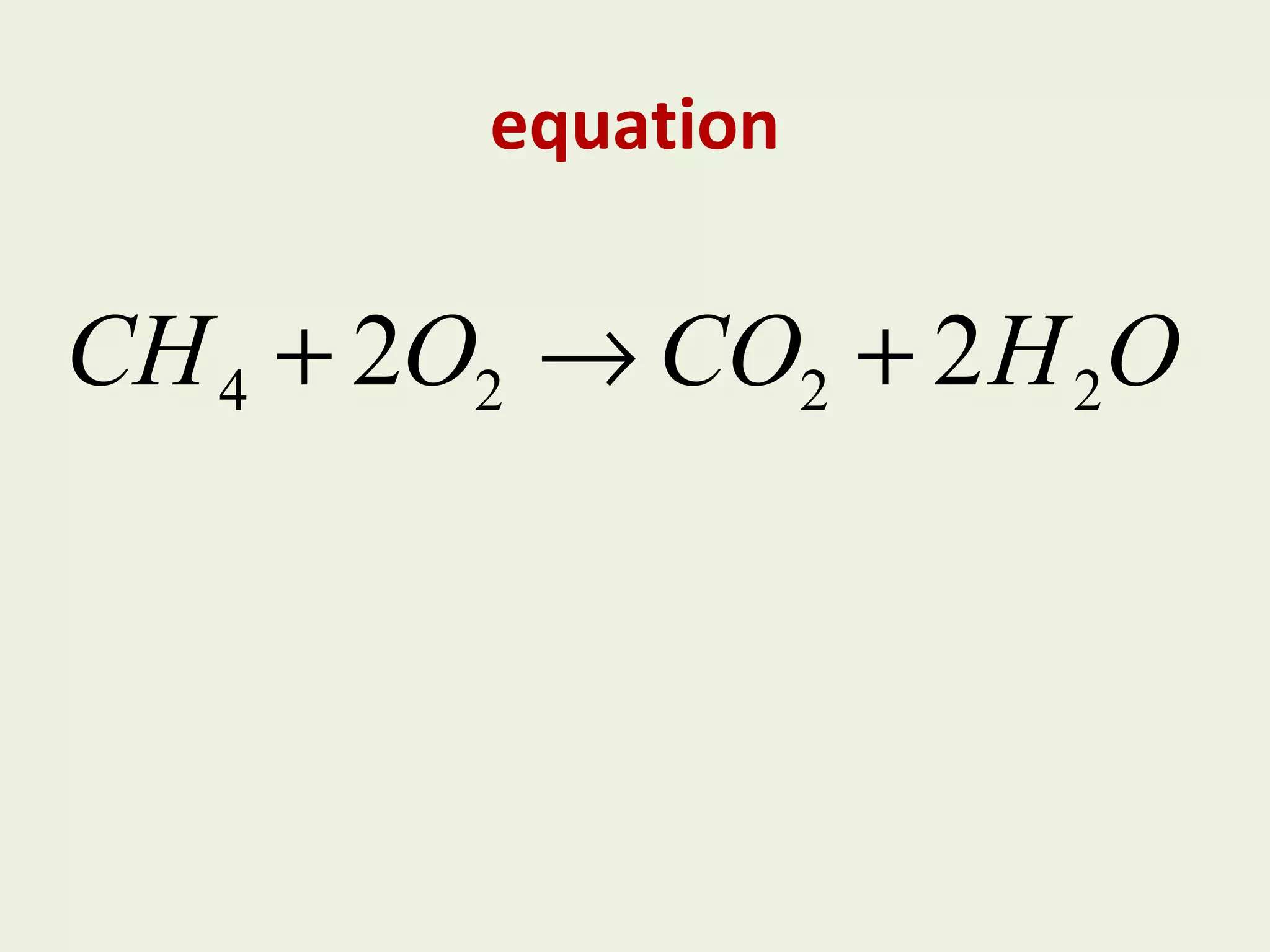Embed presentation
Downloaded 154 times


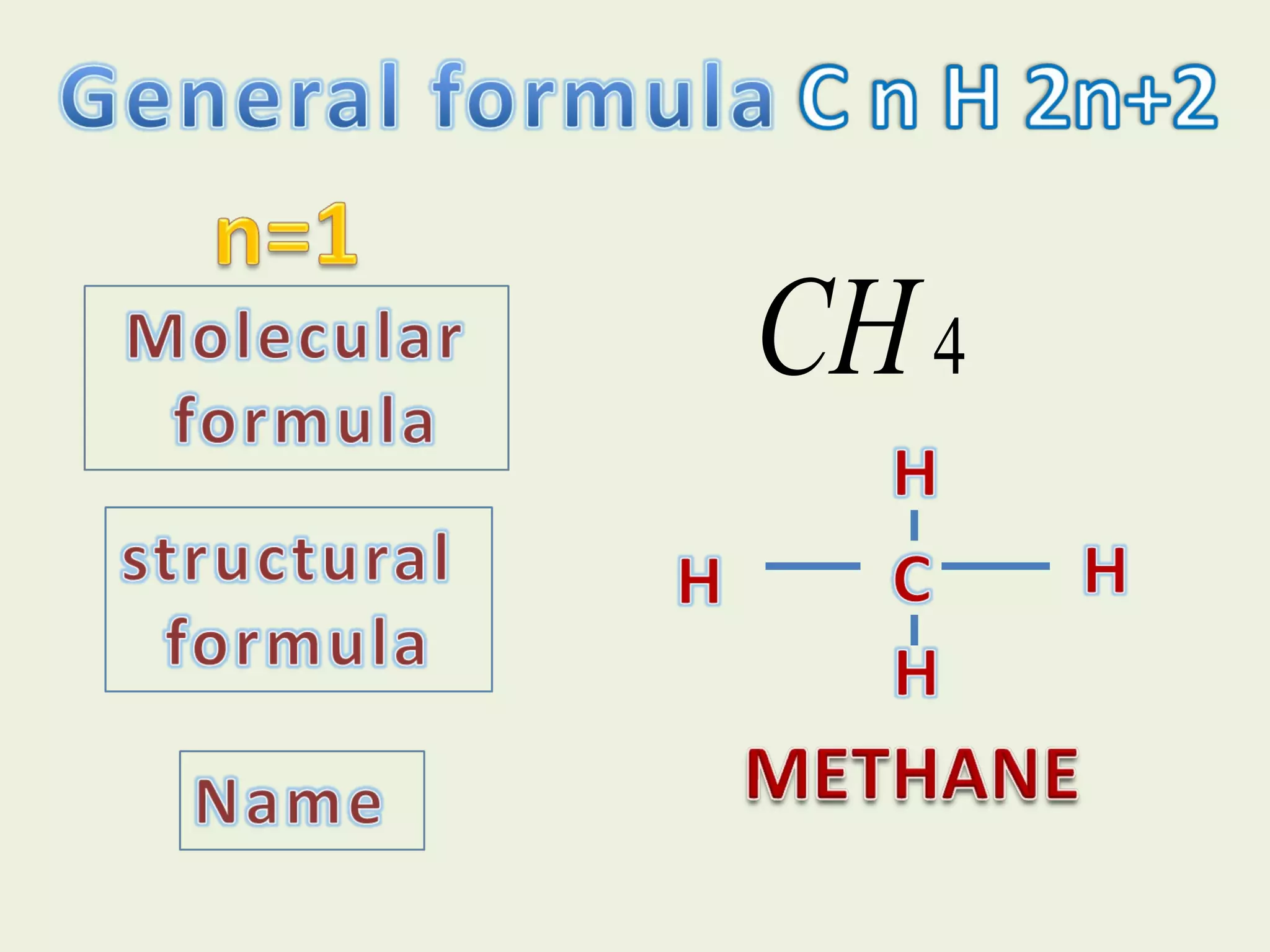
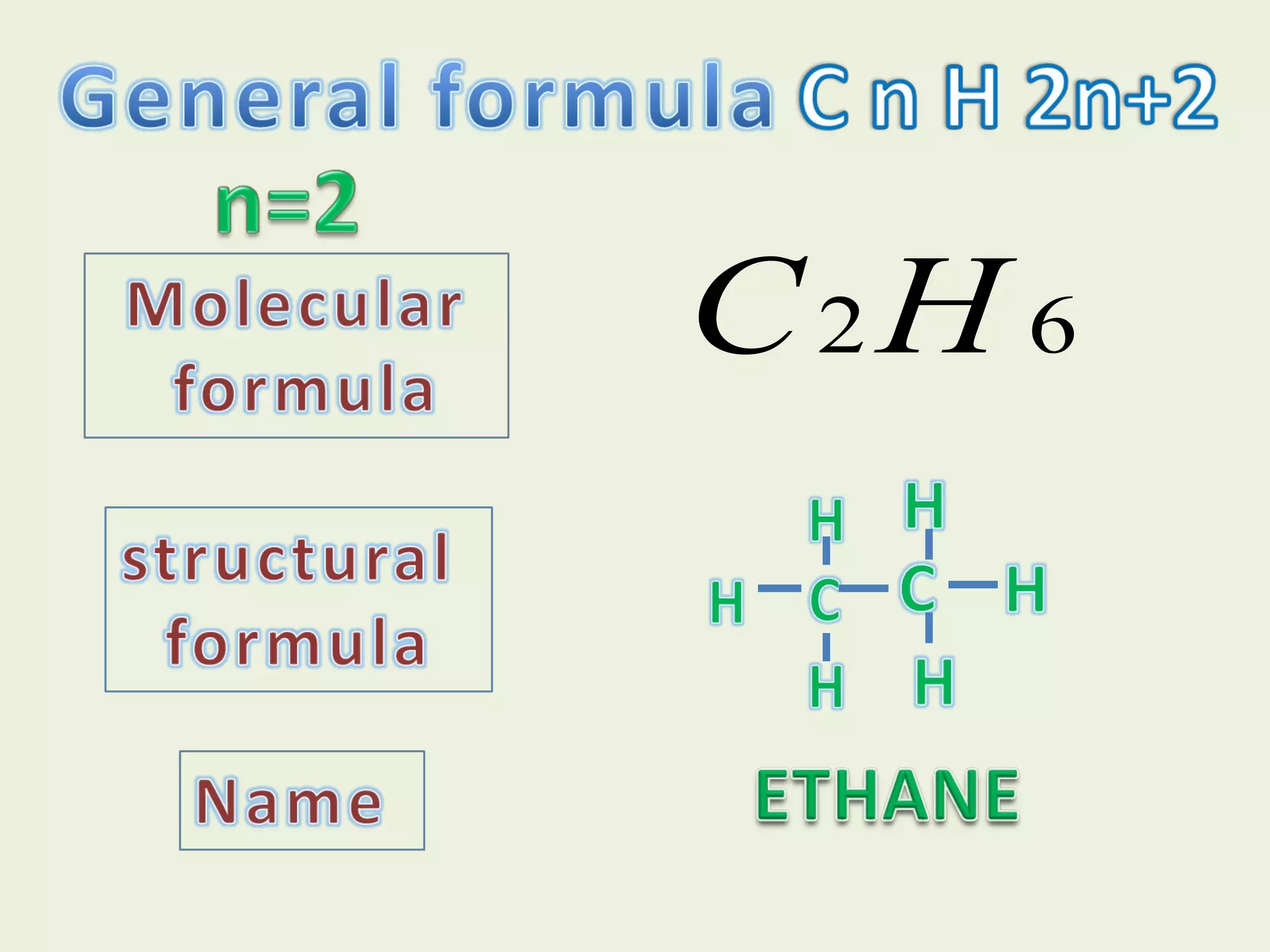
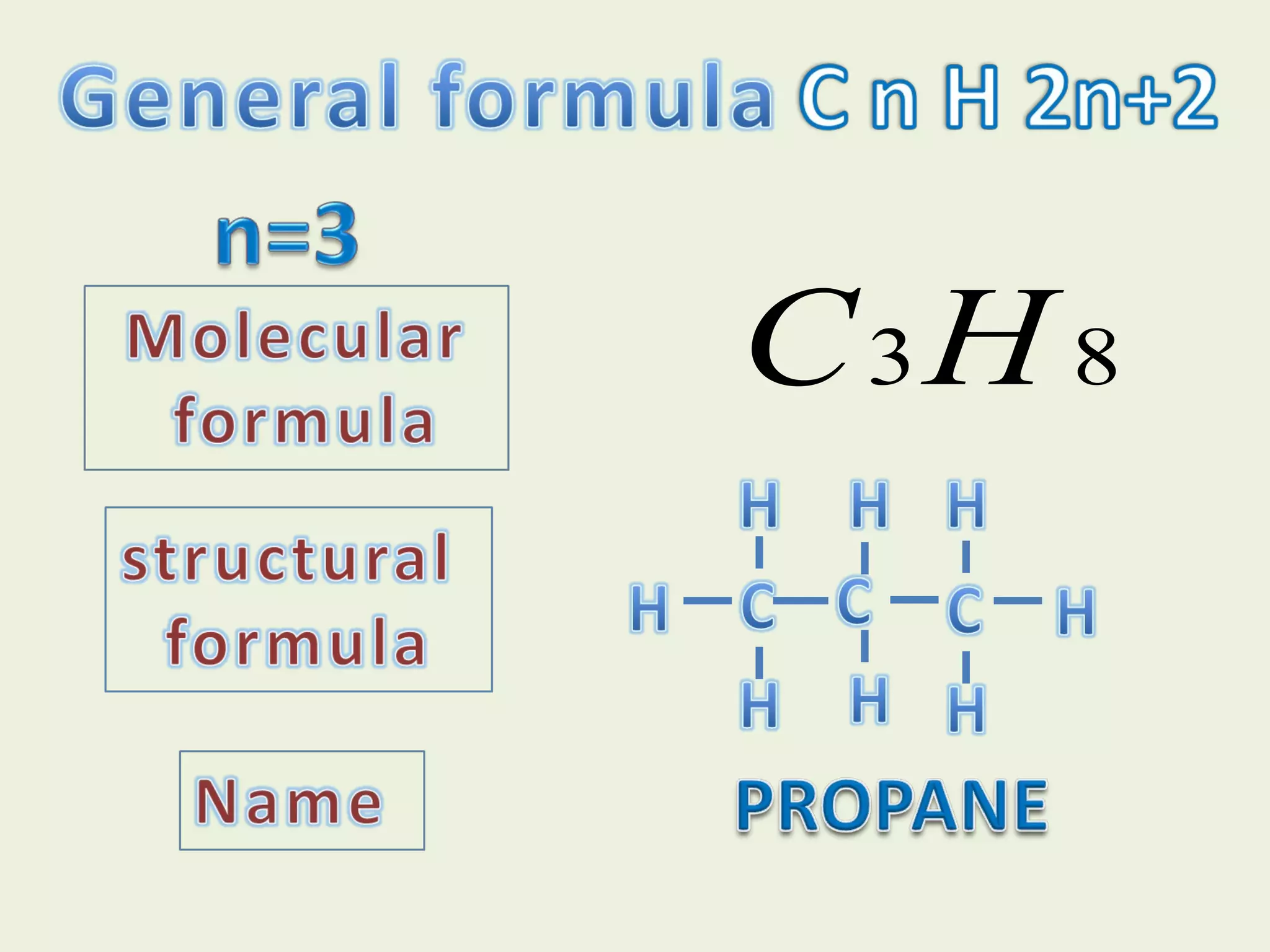
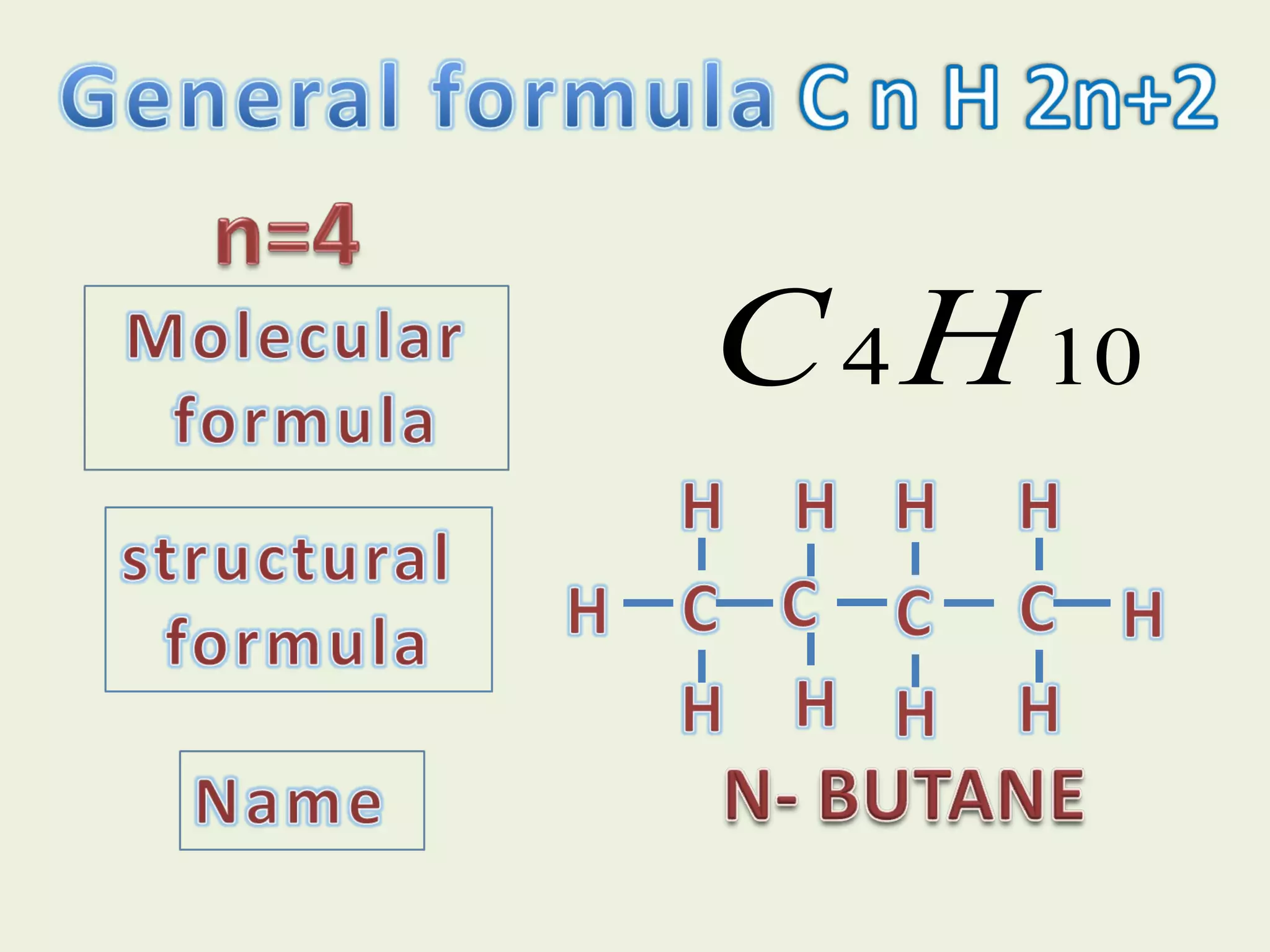
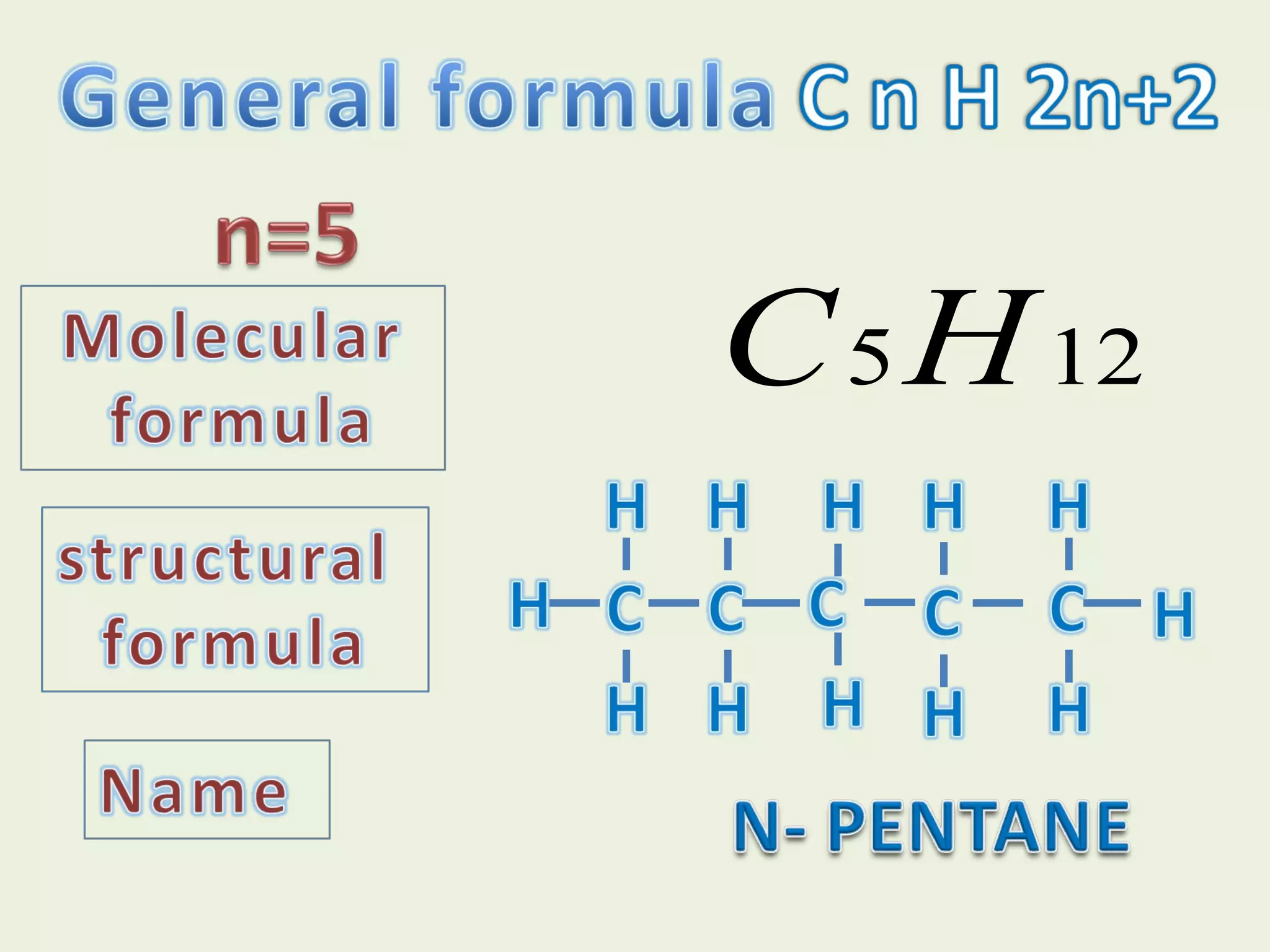
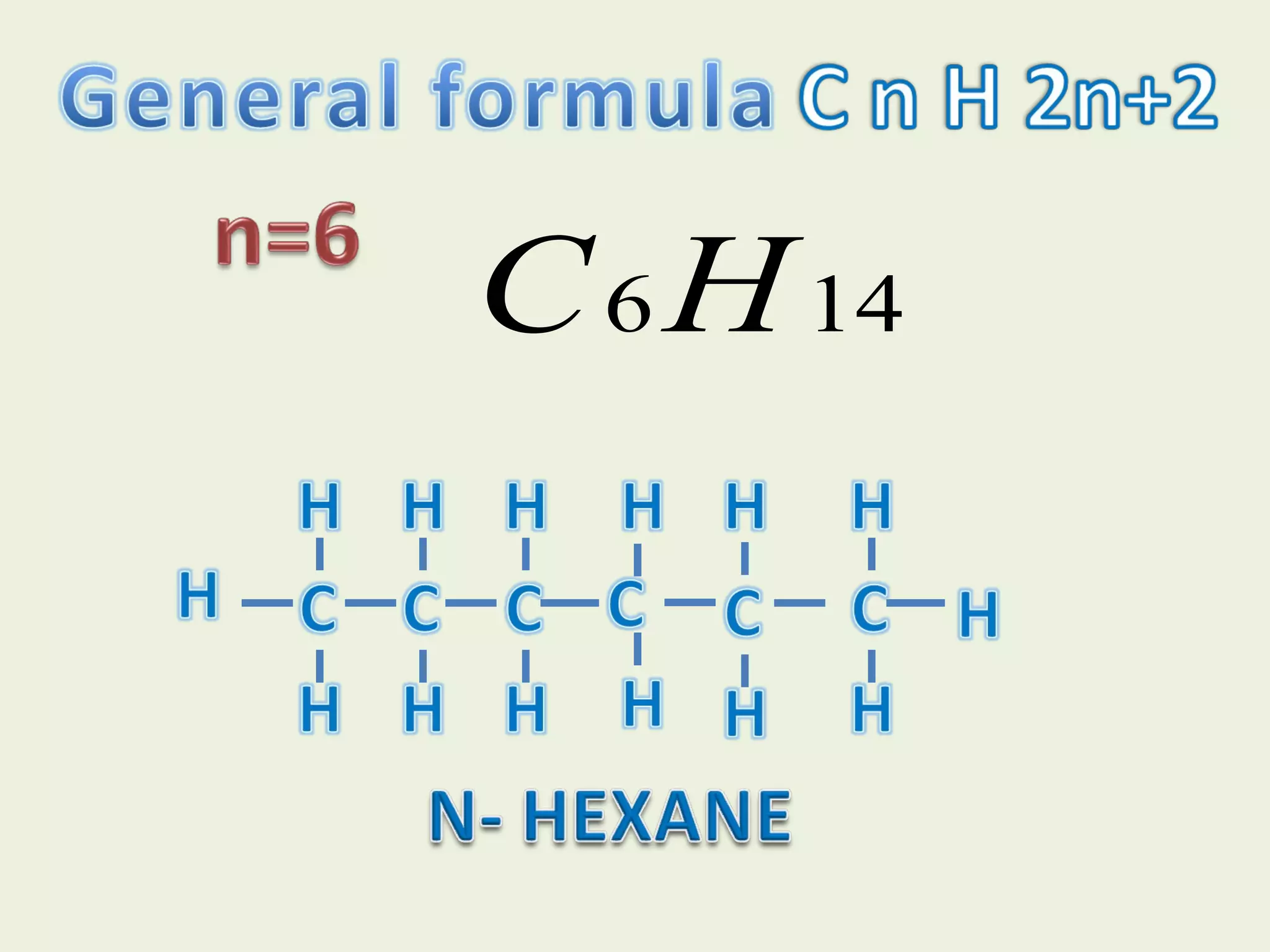
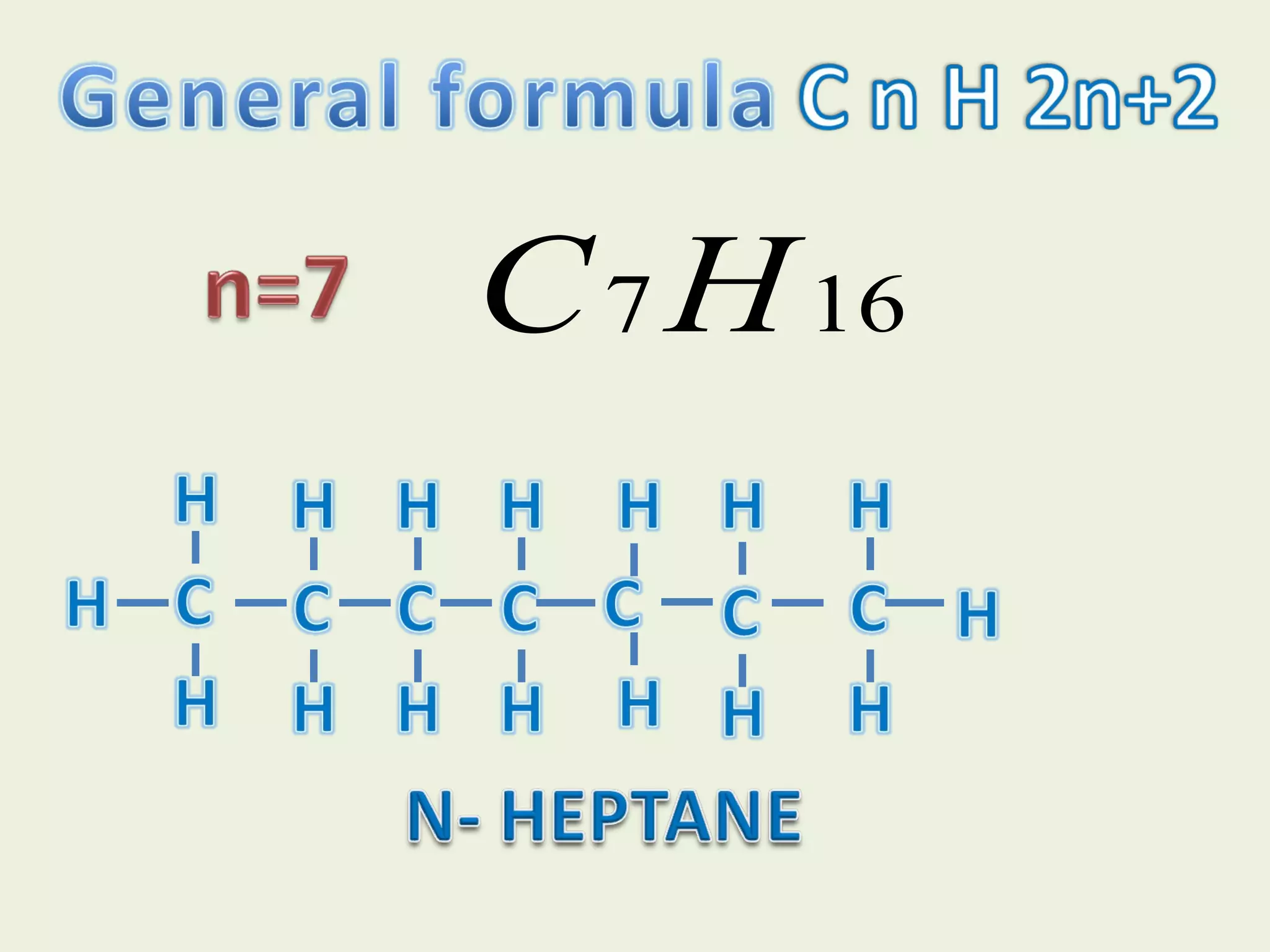
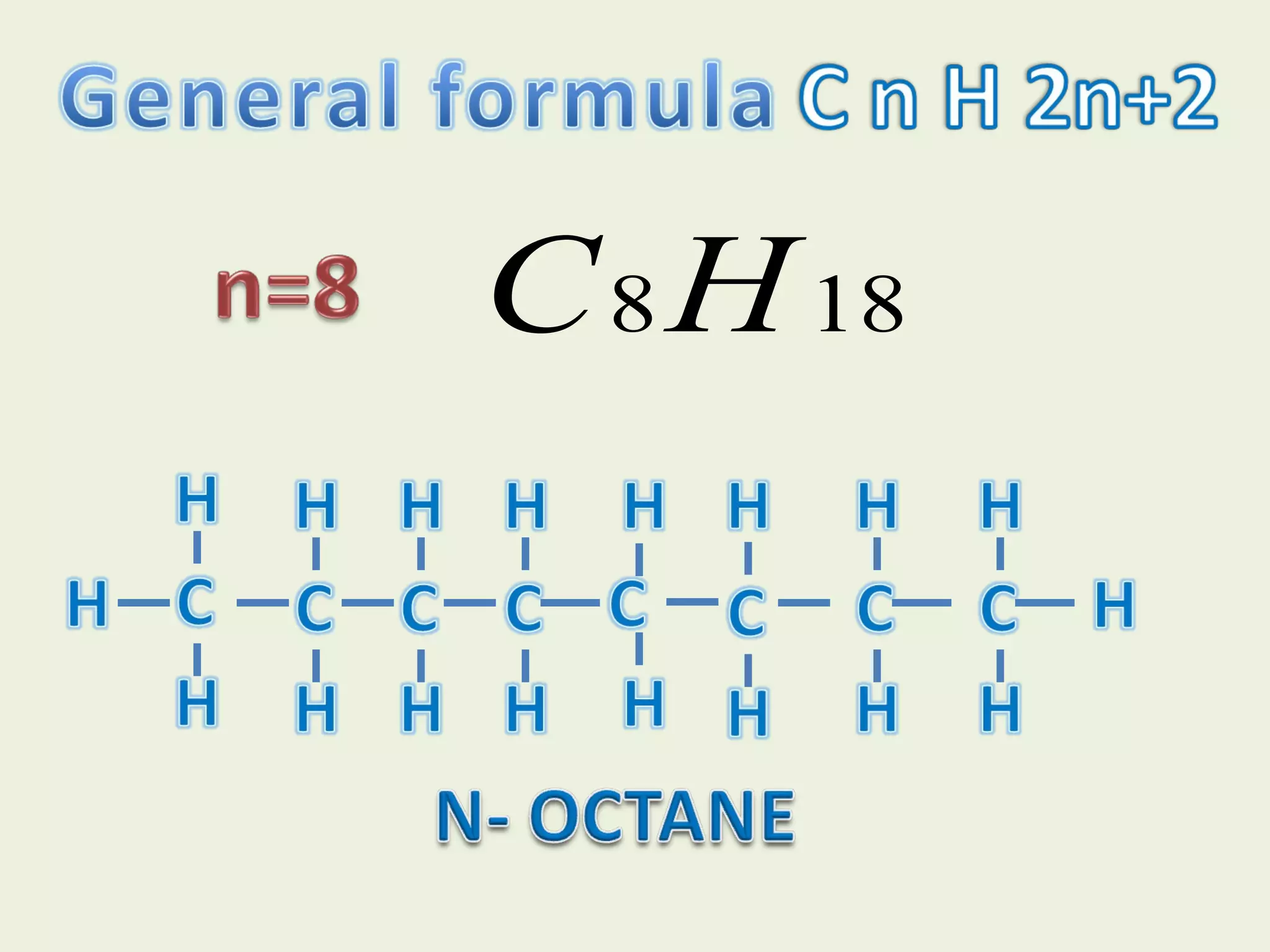
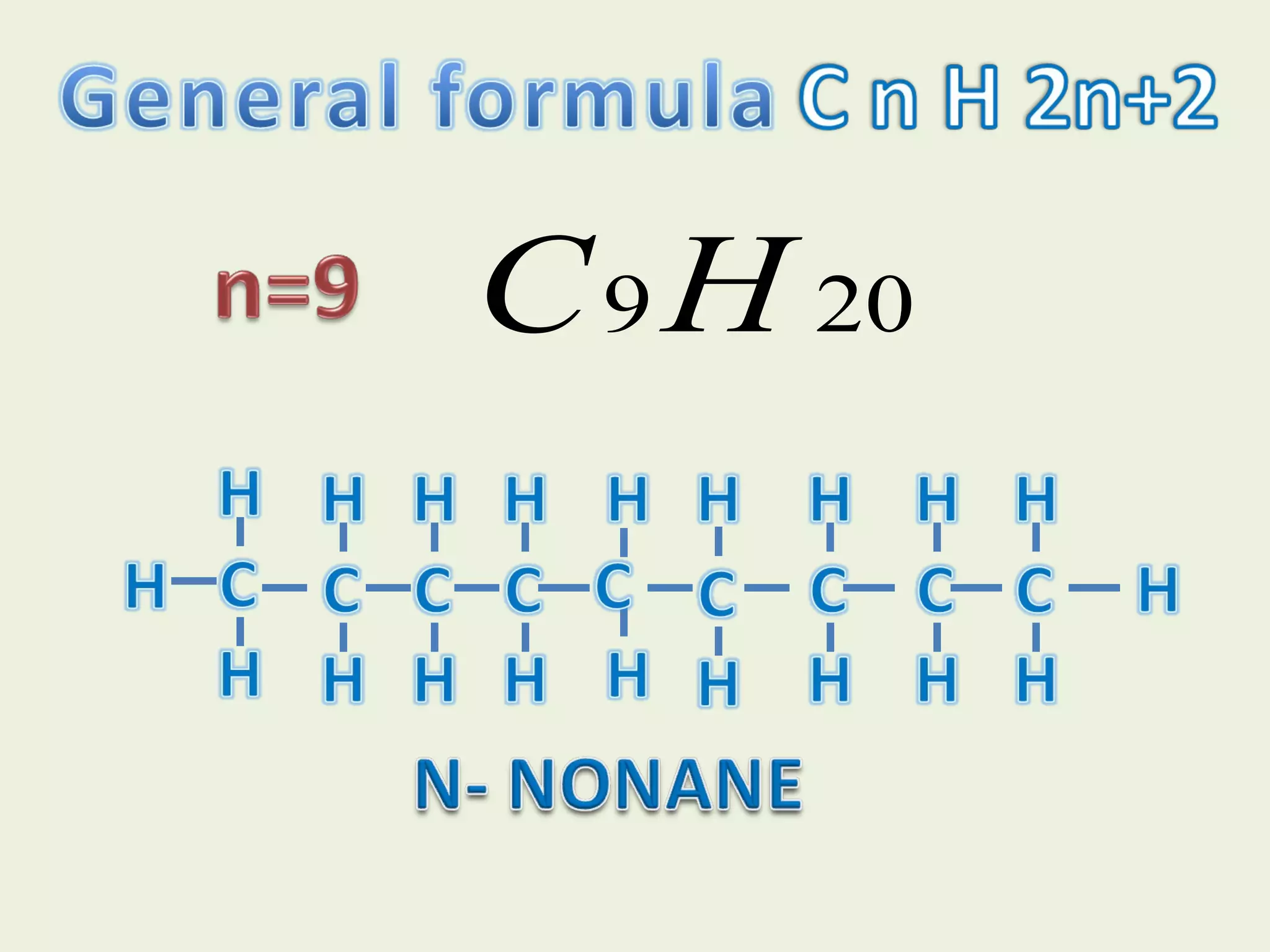
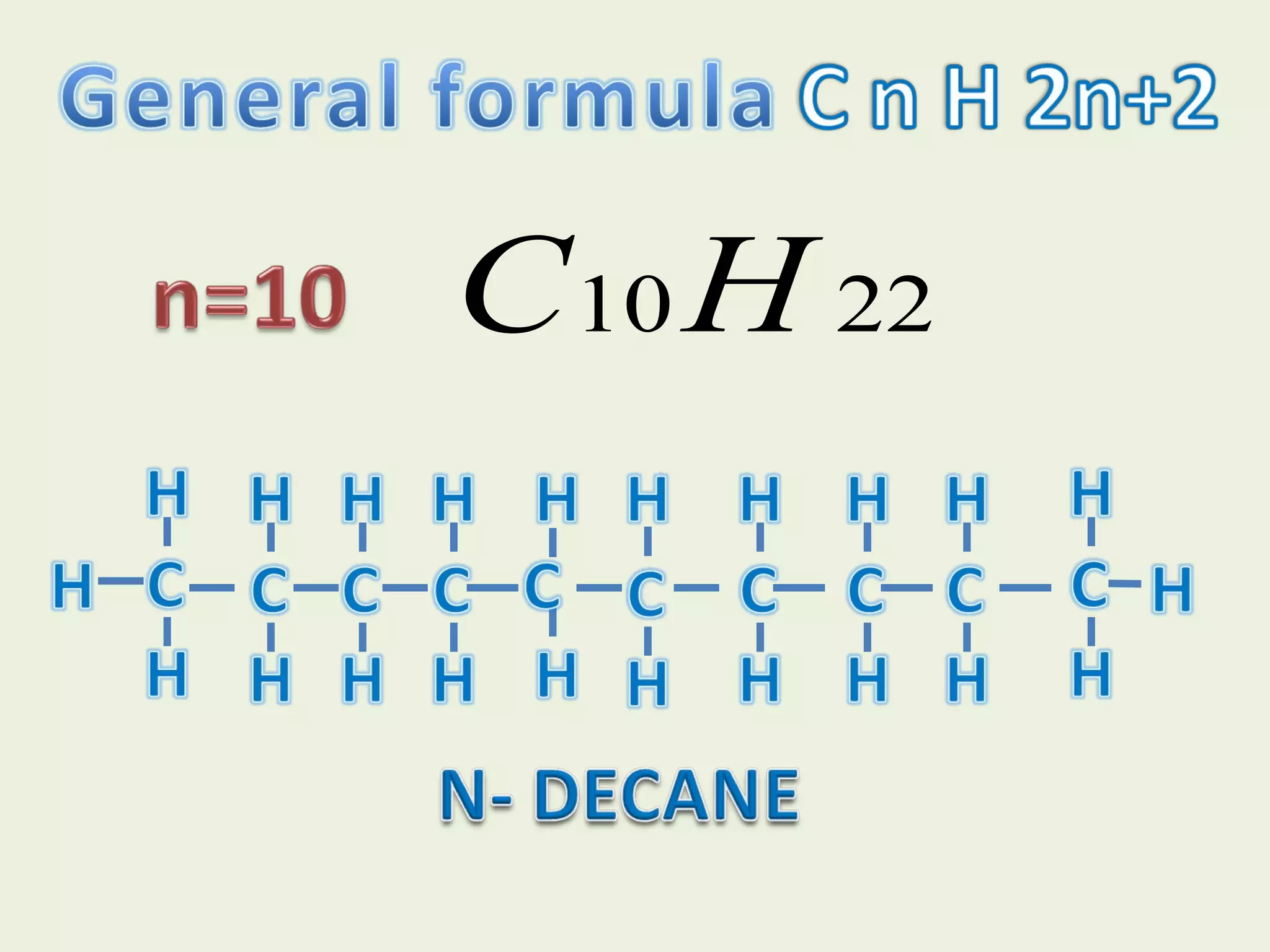

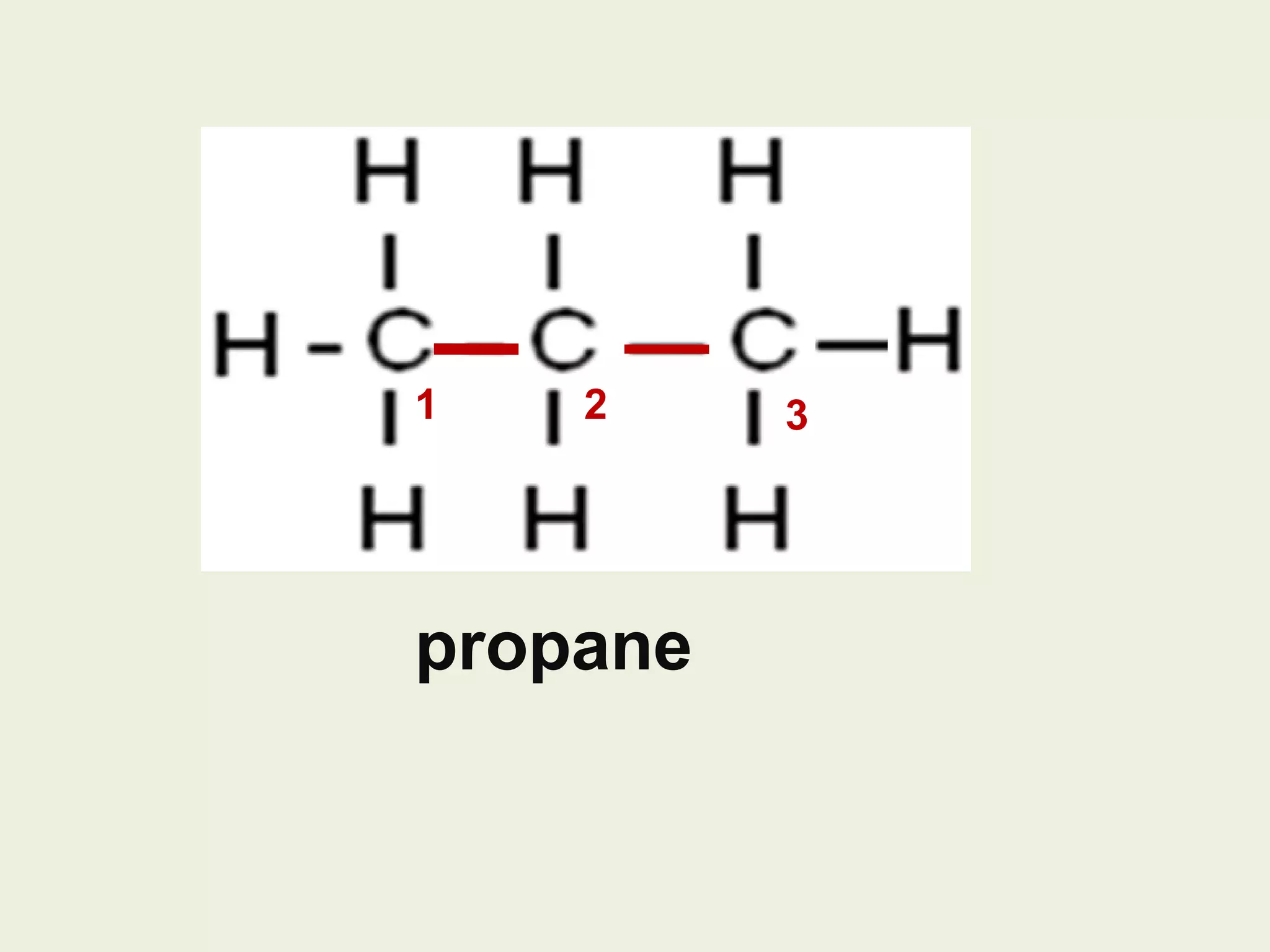
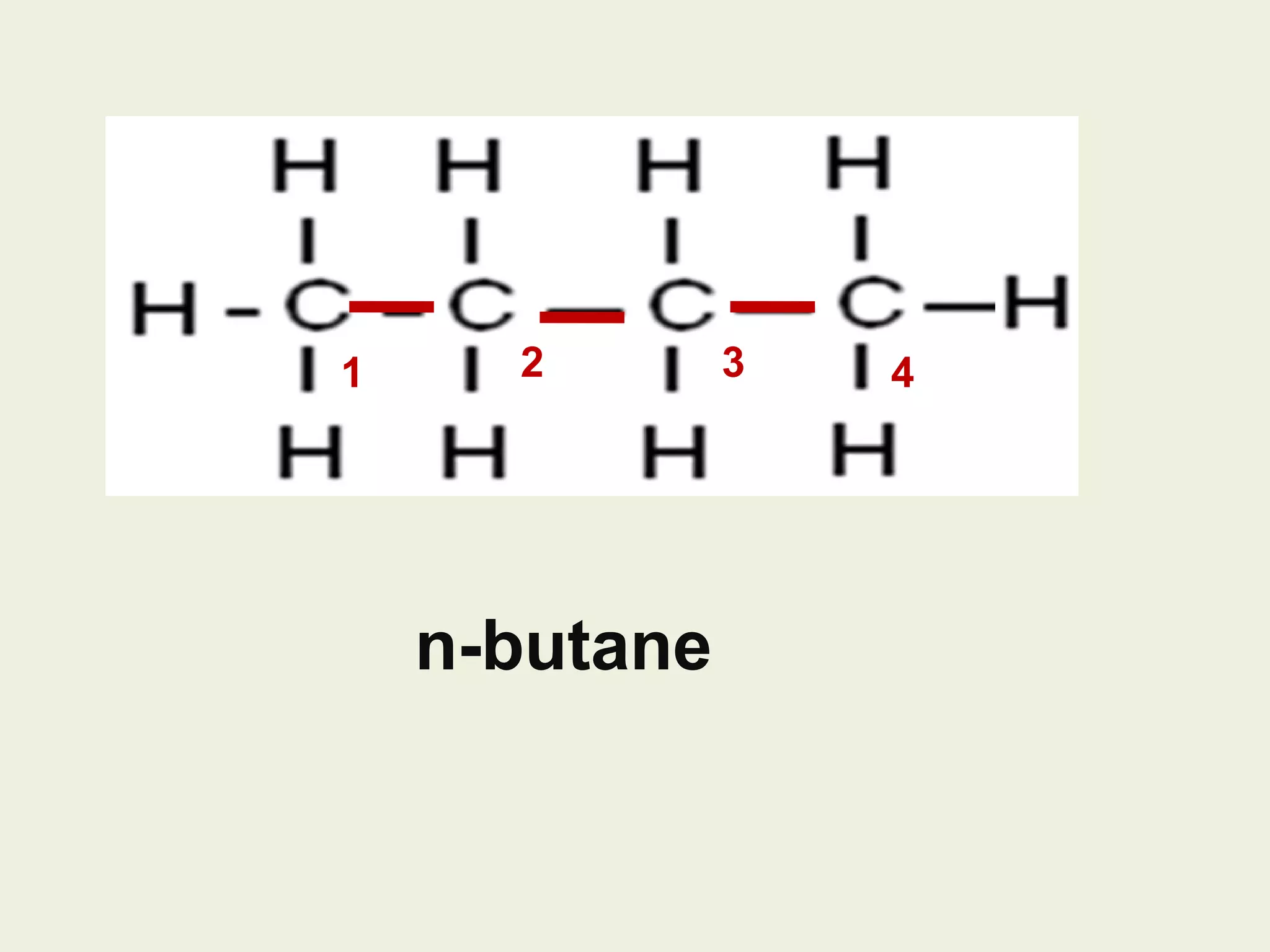
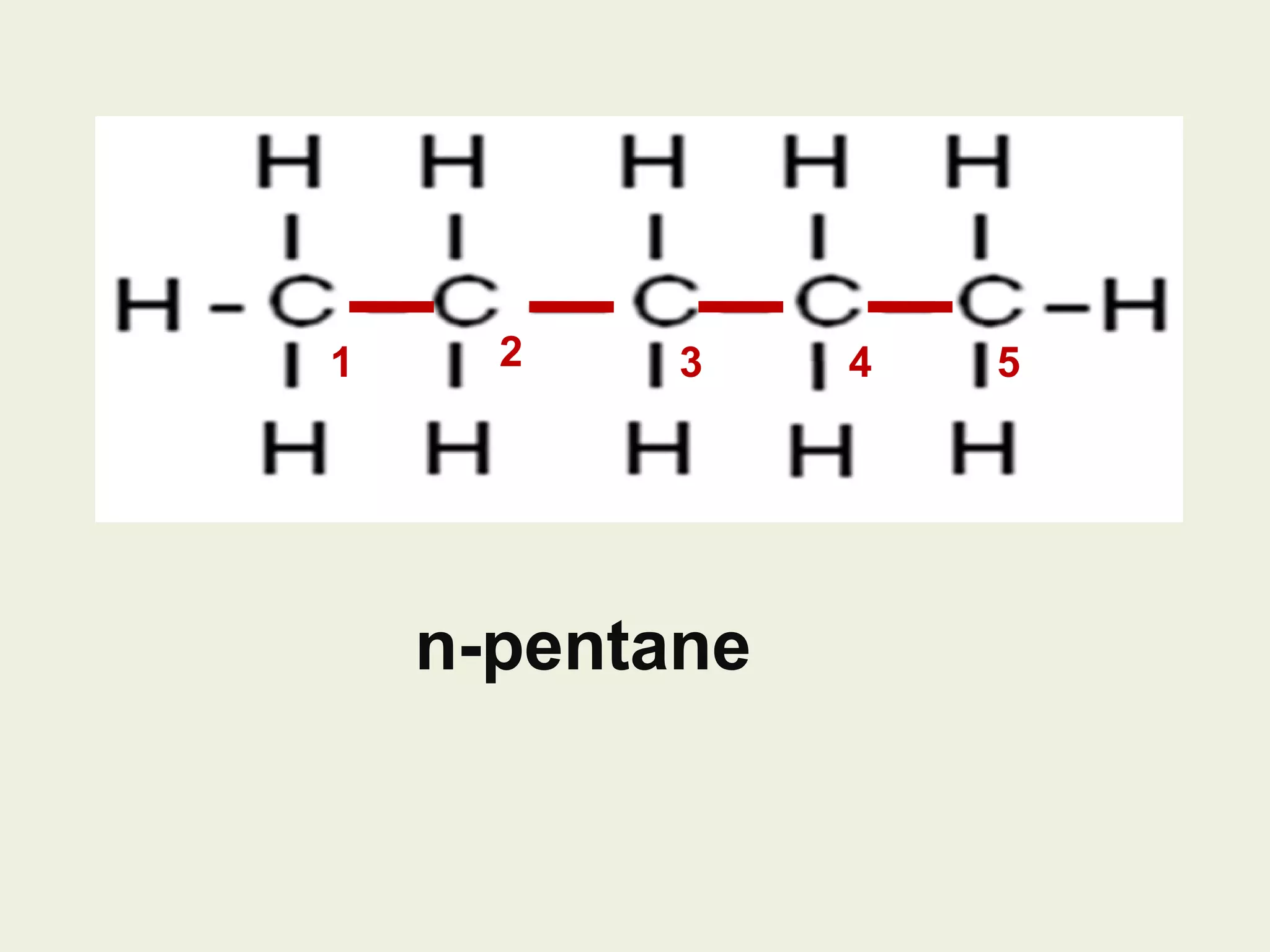

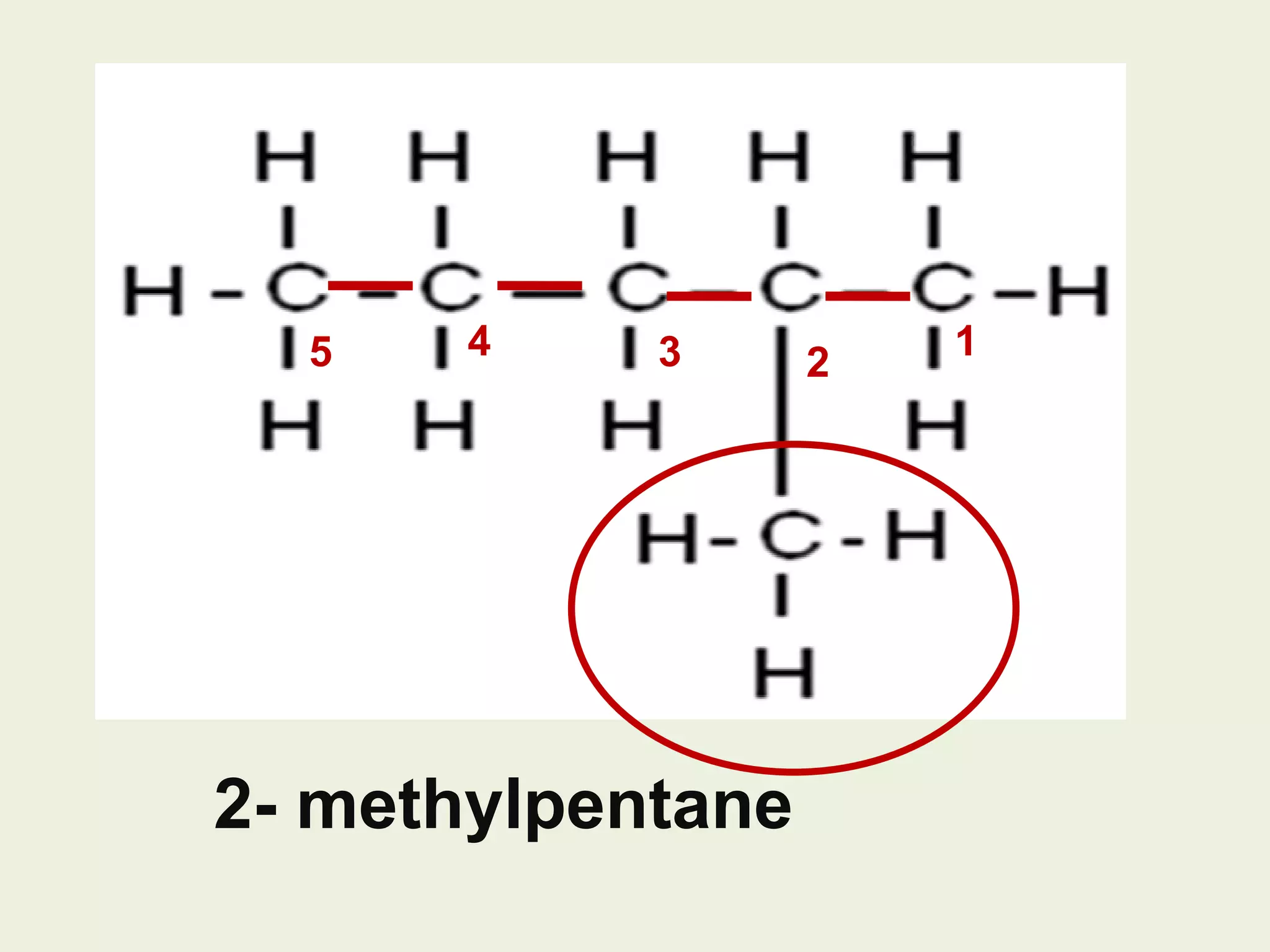
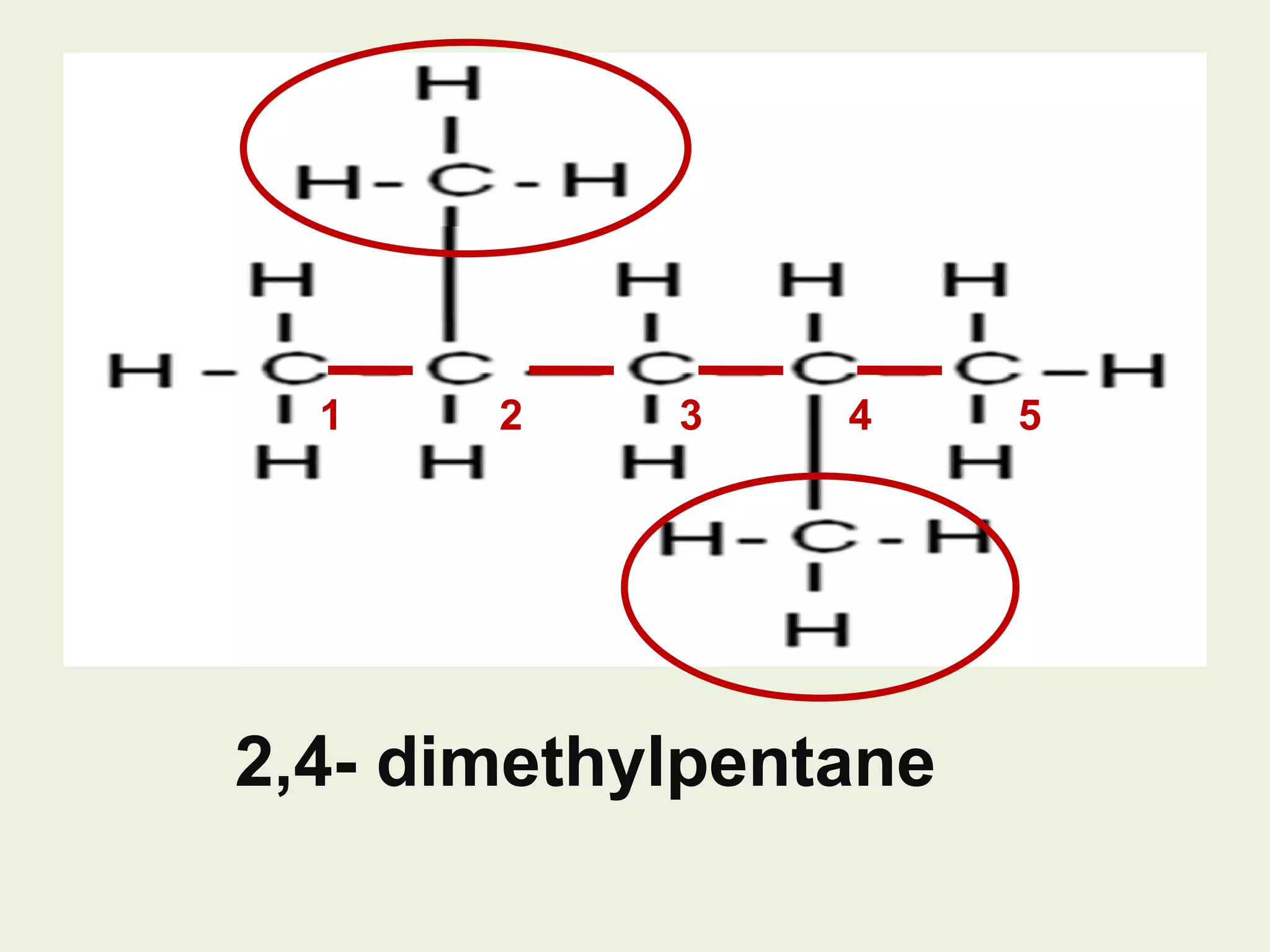



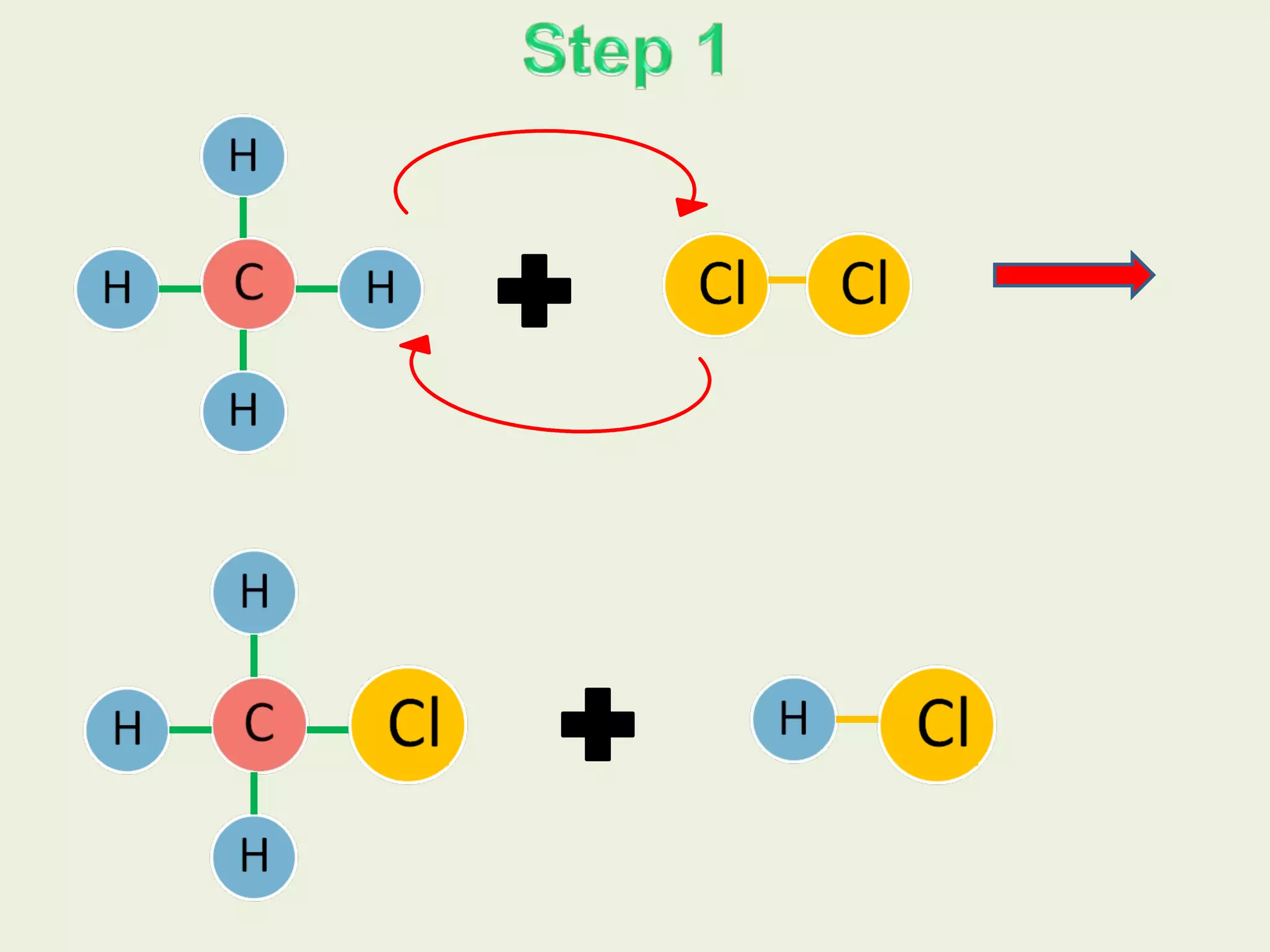
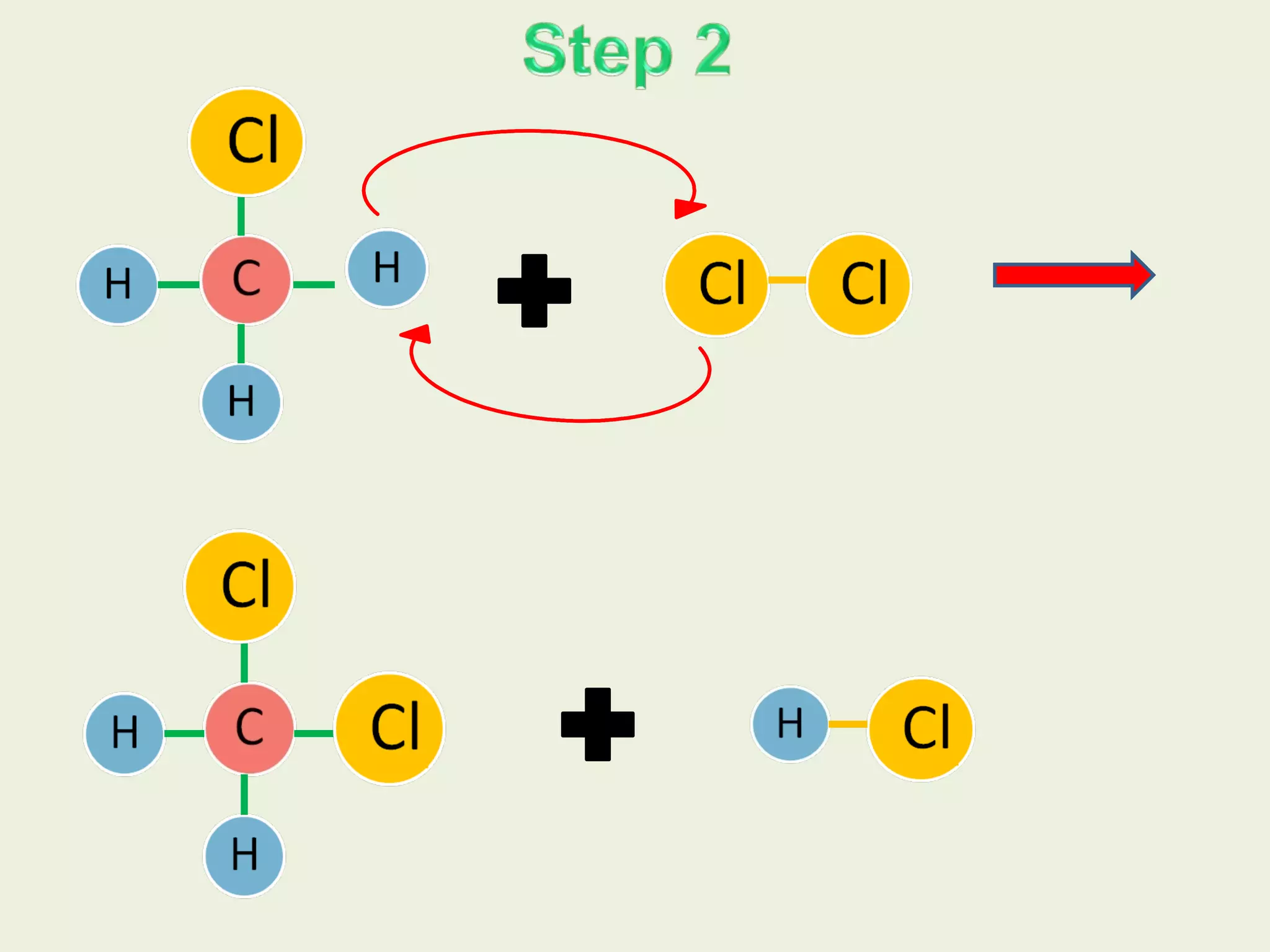
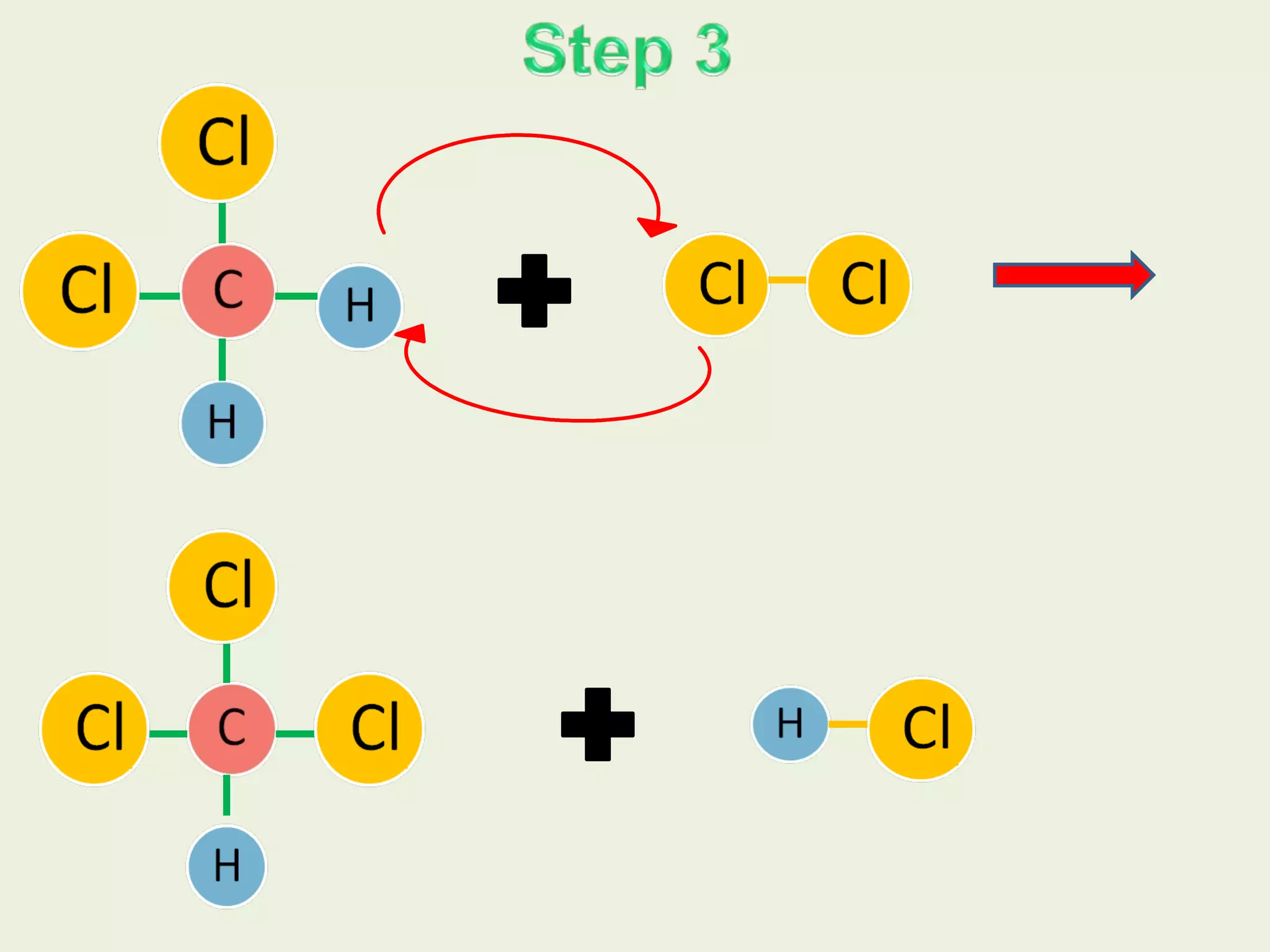
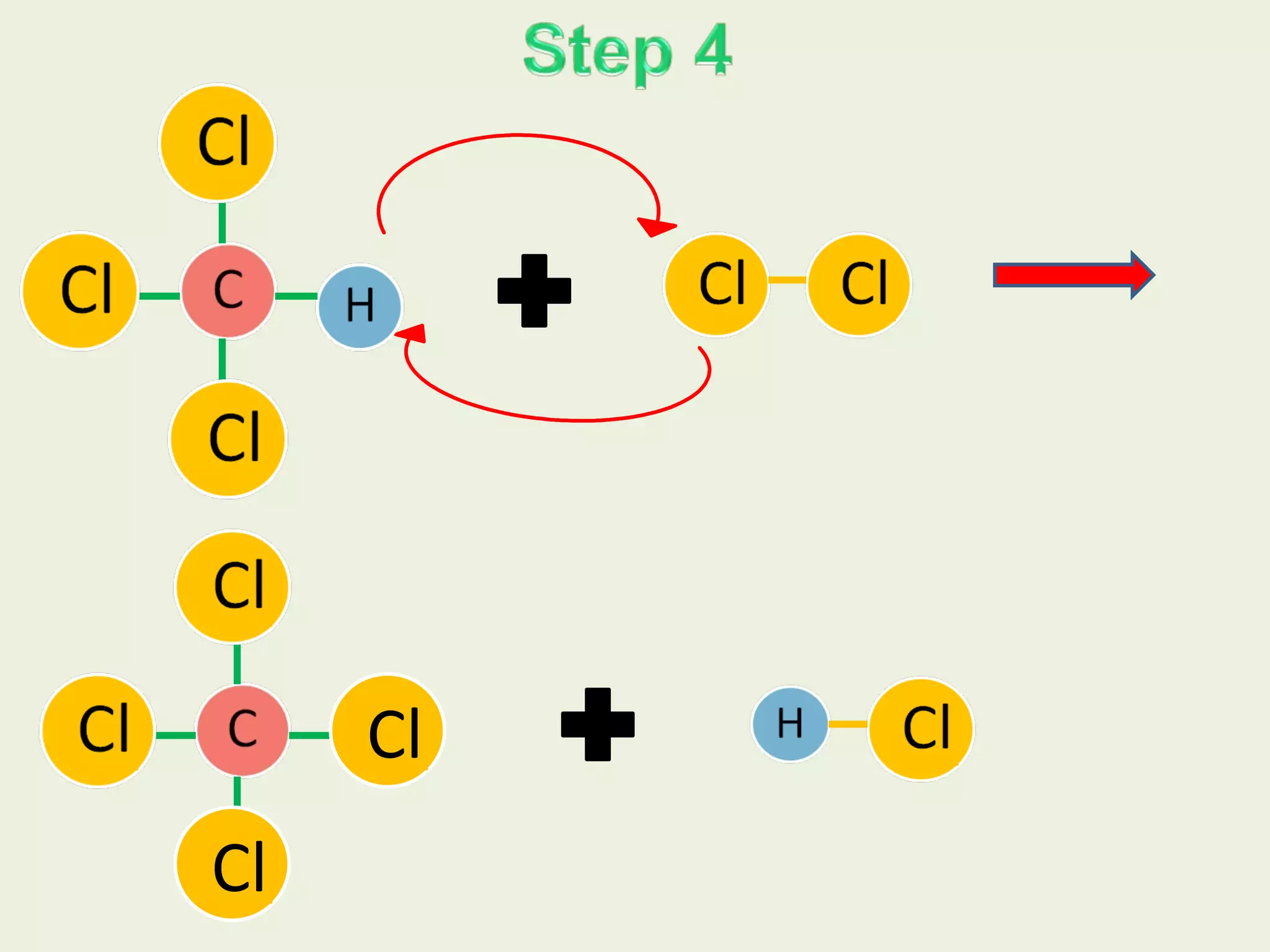
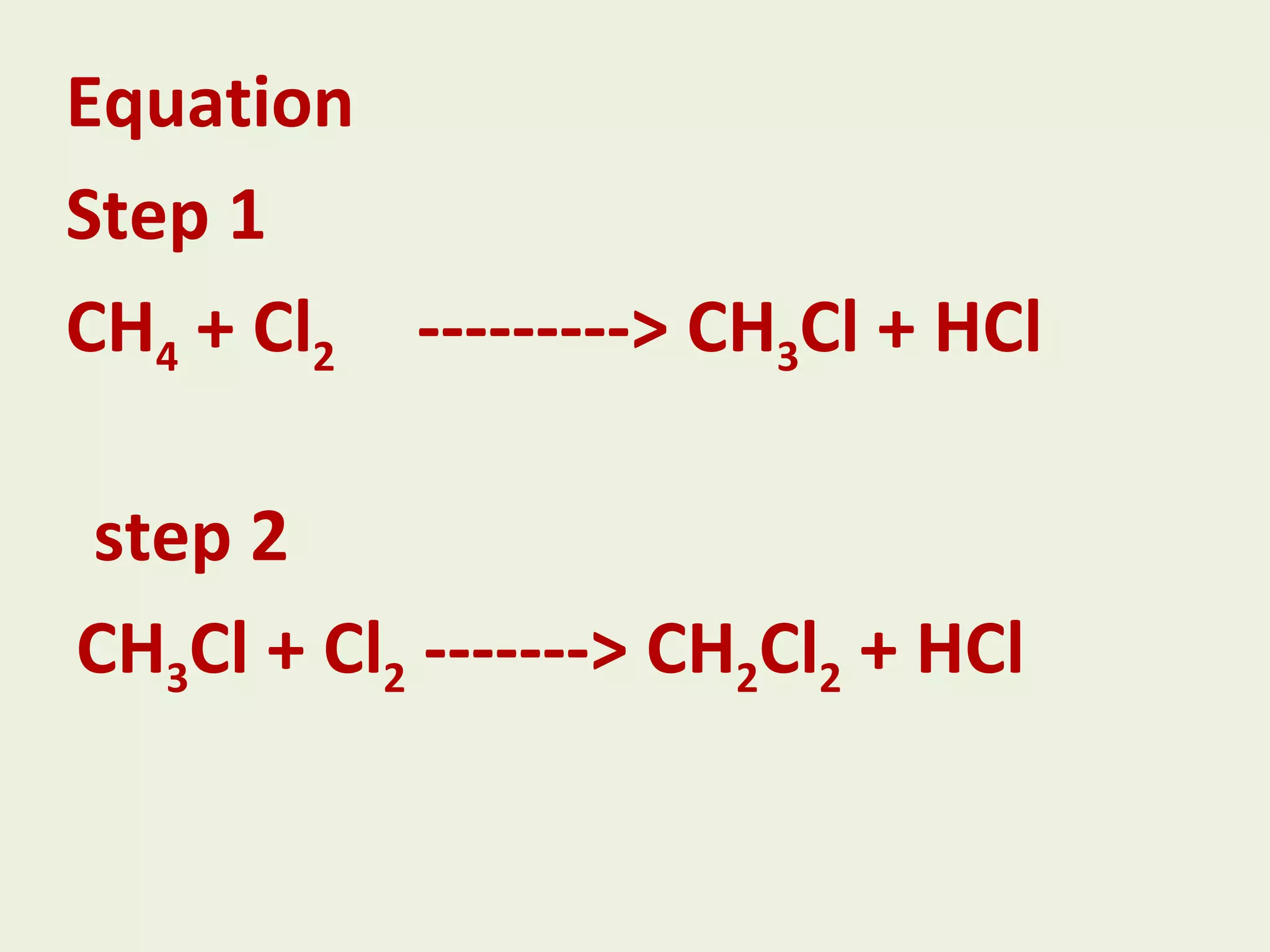
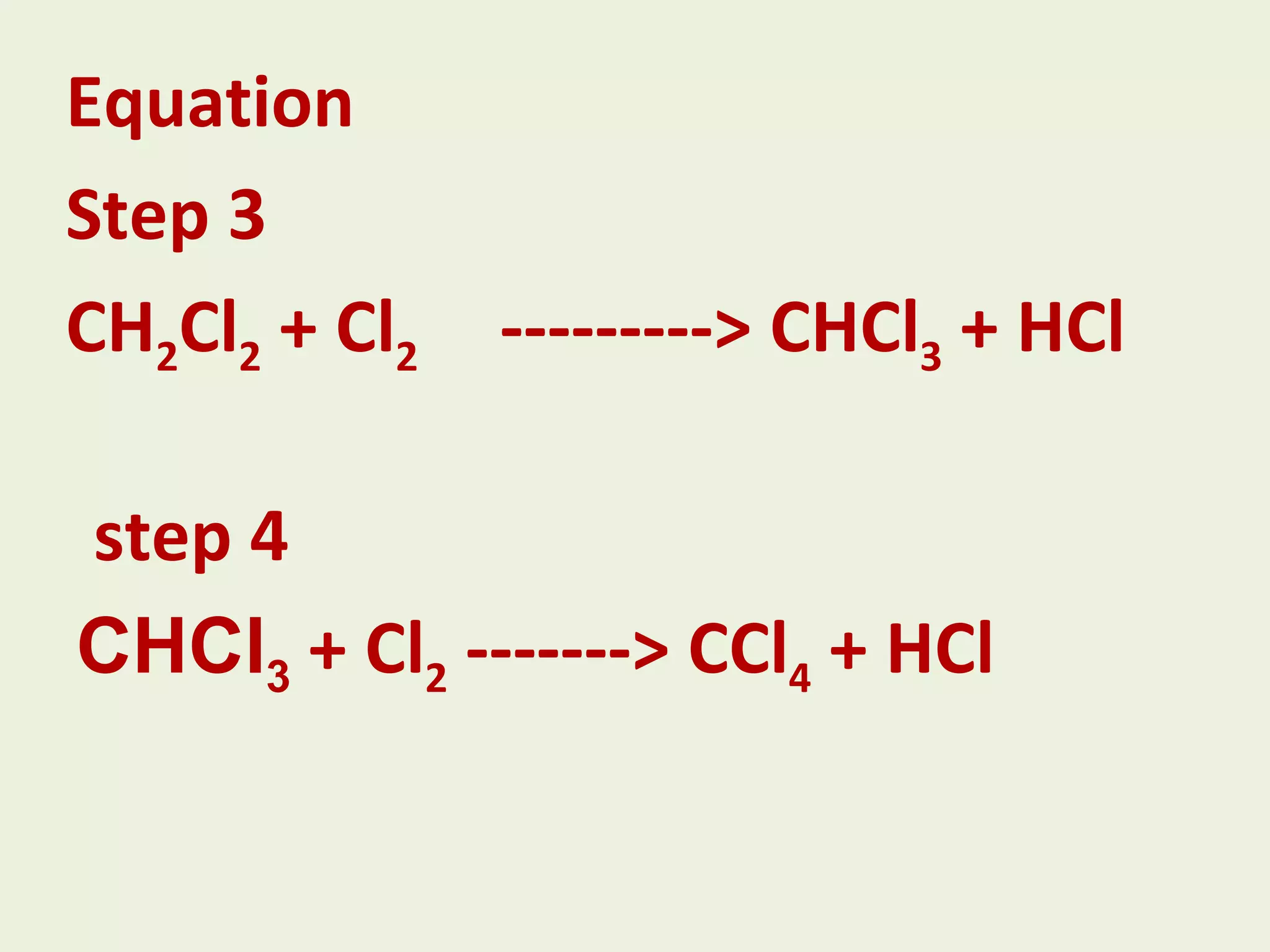

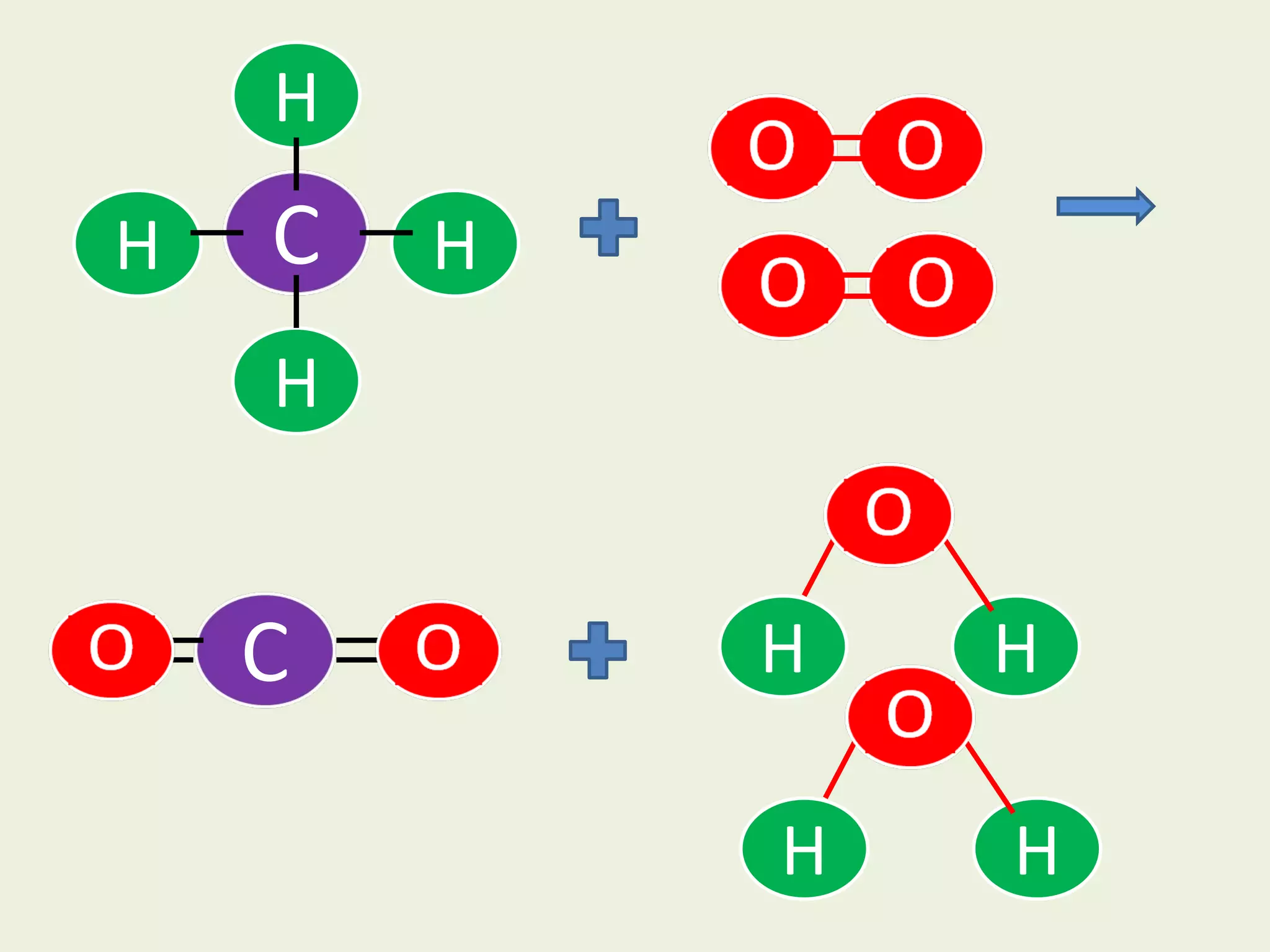
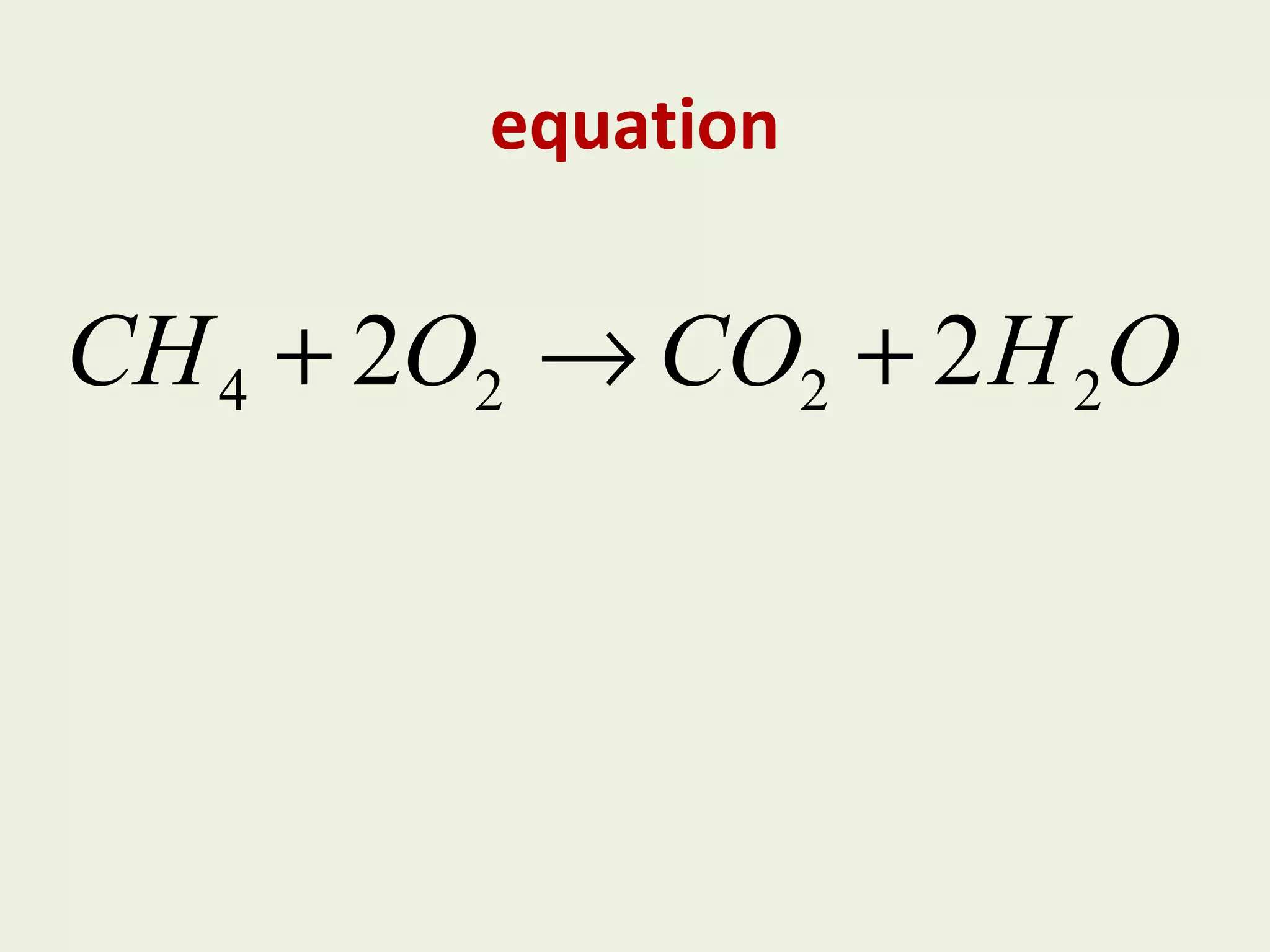


This document discusses alkanes, including their general formula, molecular formula, structural formula, and naming conventions. It provides examples of how to name alkanes based on identifying the stem and any branches, then numbering along the main chain. The document also briefly outlines some physical properties of alkanes and two of their chemical properties - combustion and substitution reactions, giving examples of methane combusting and its reaction with chlorine.