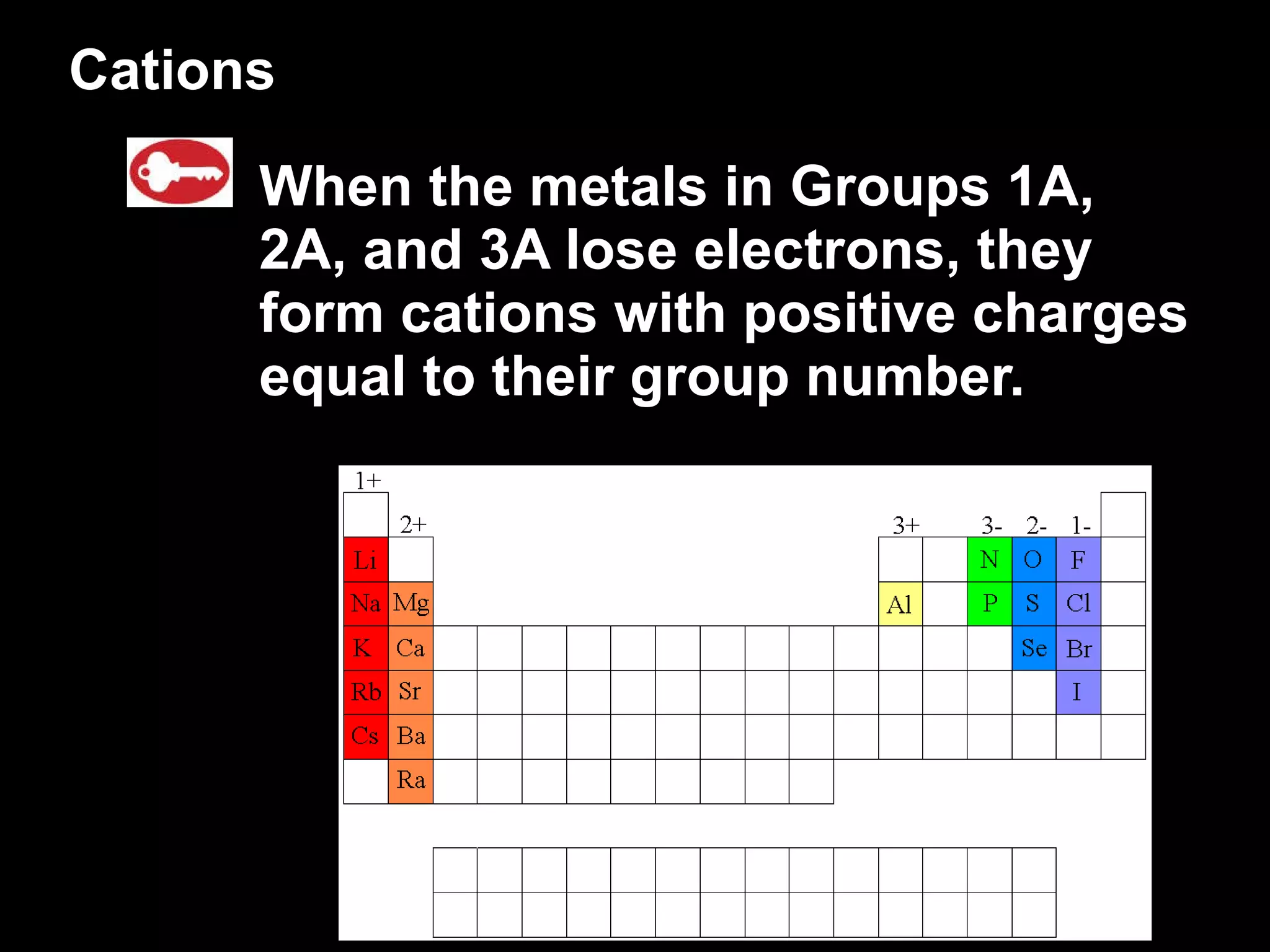
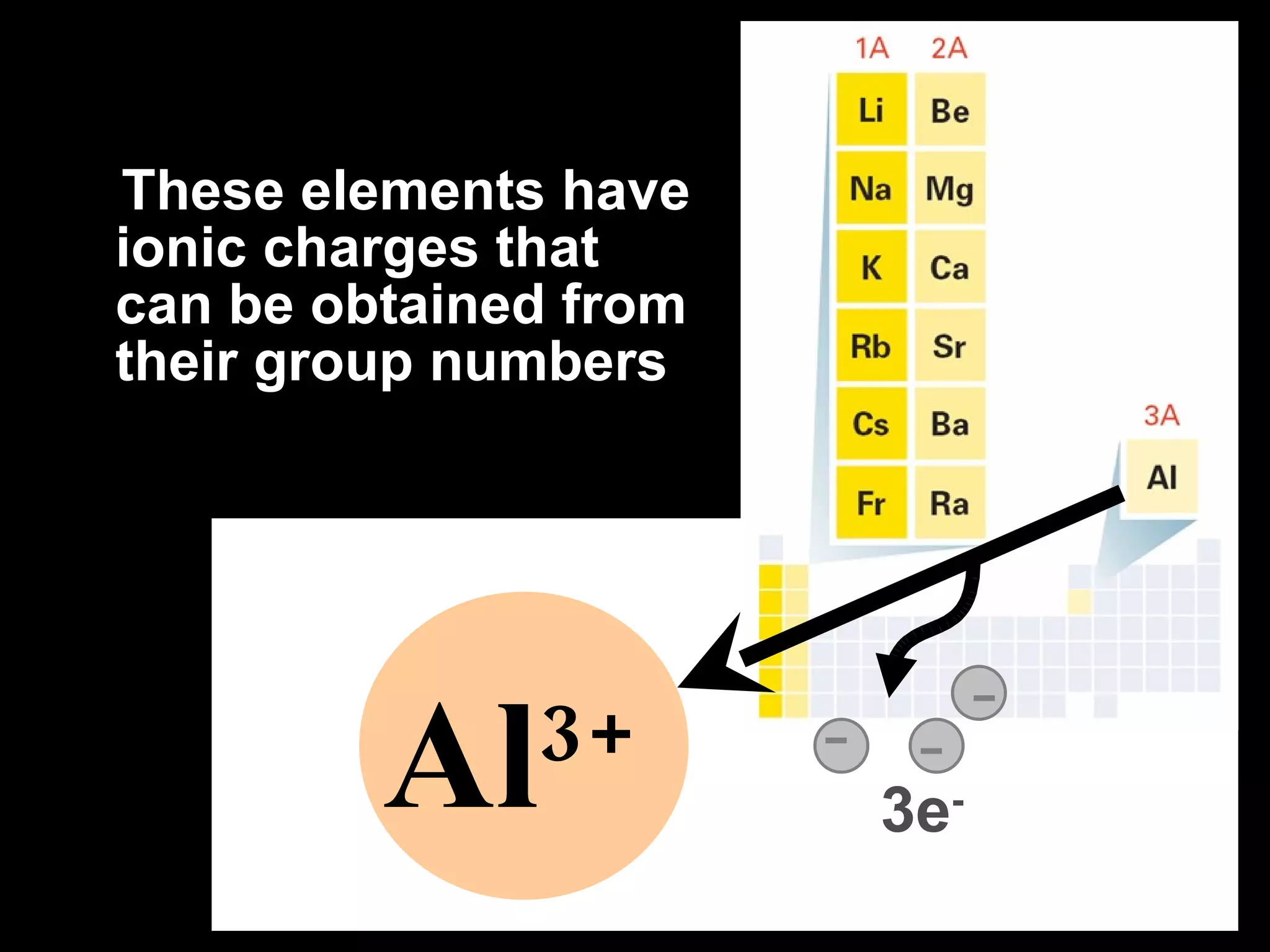
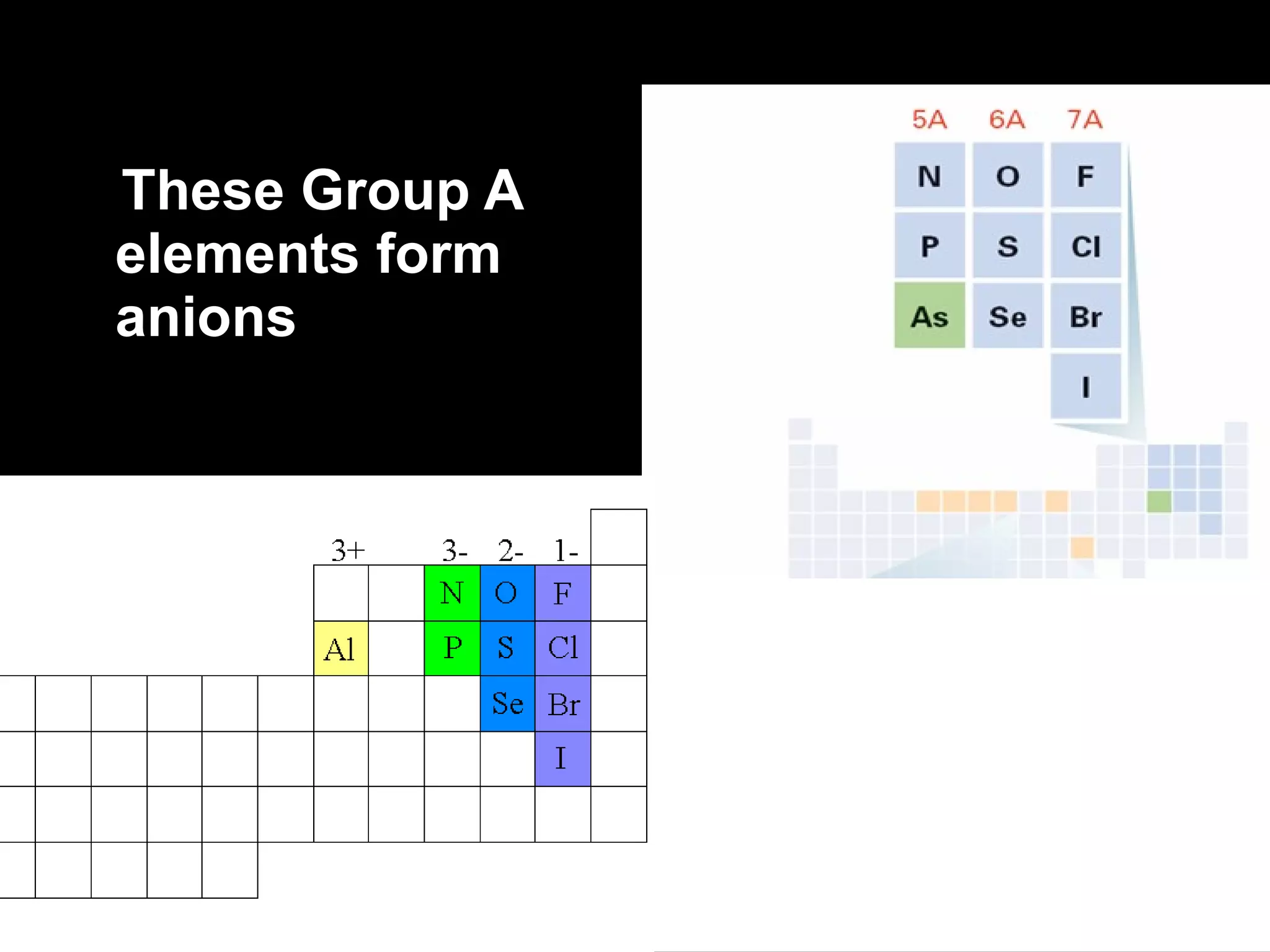
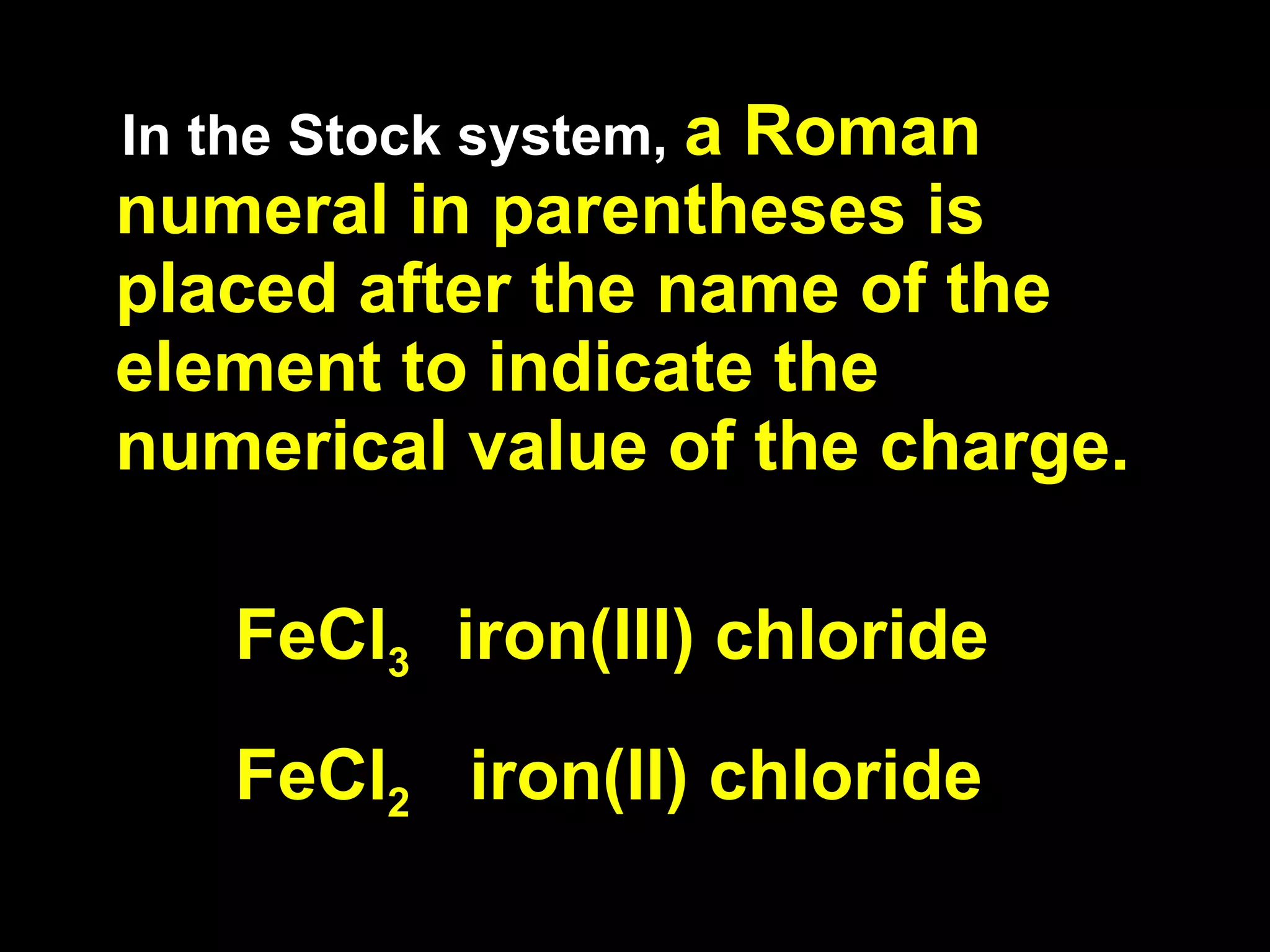
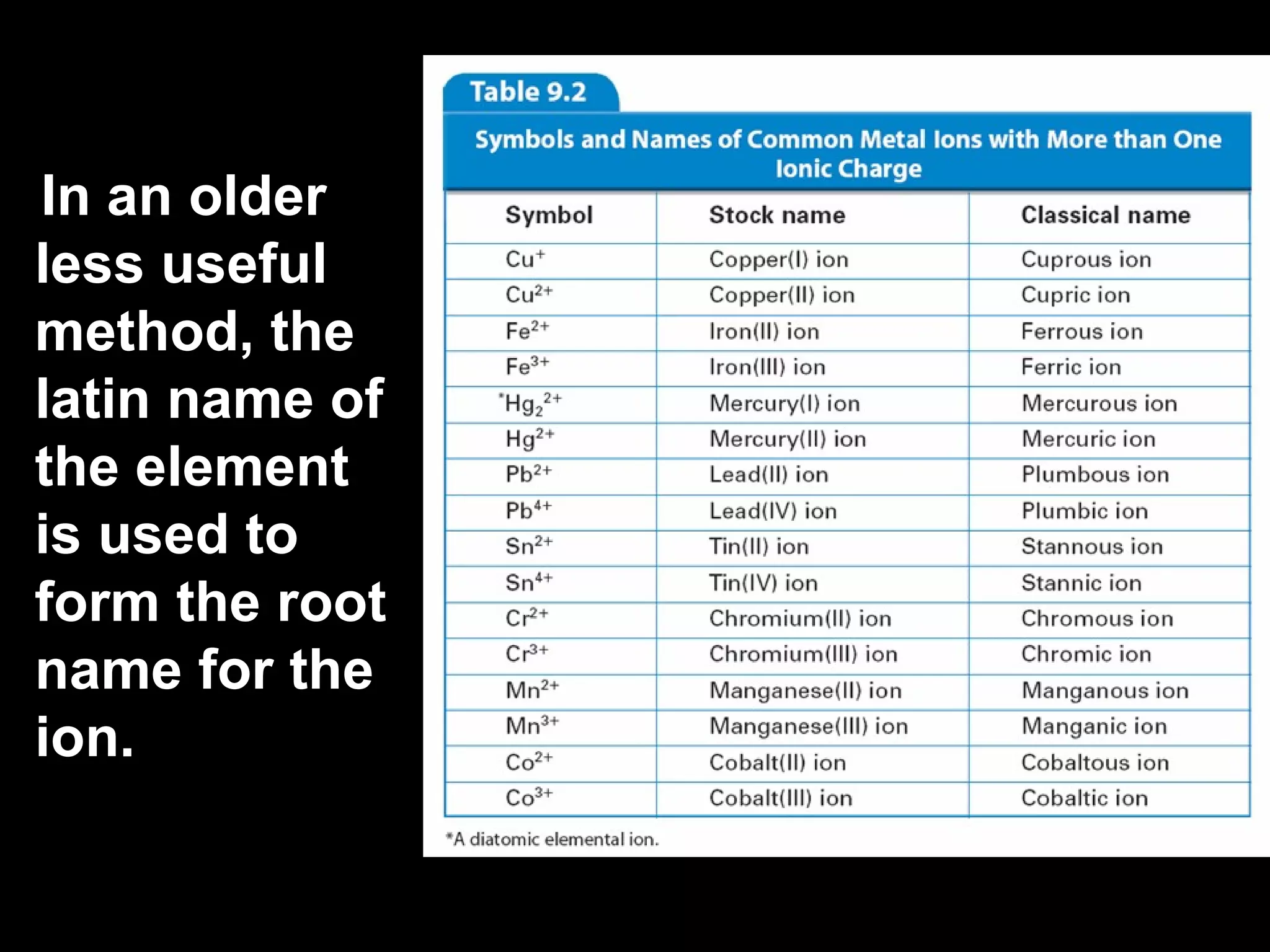
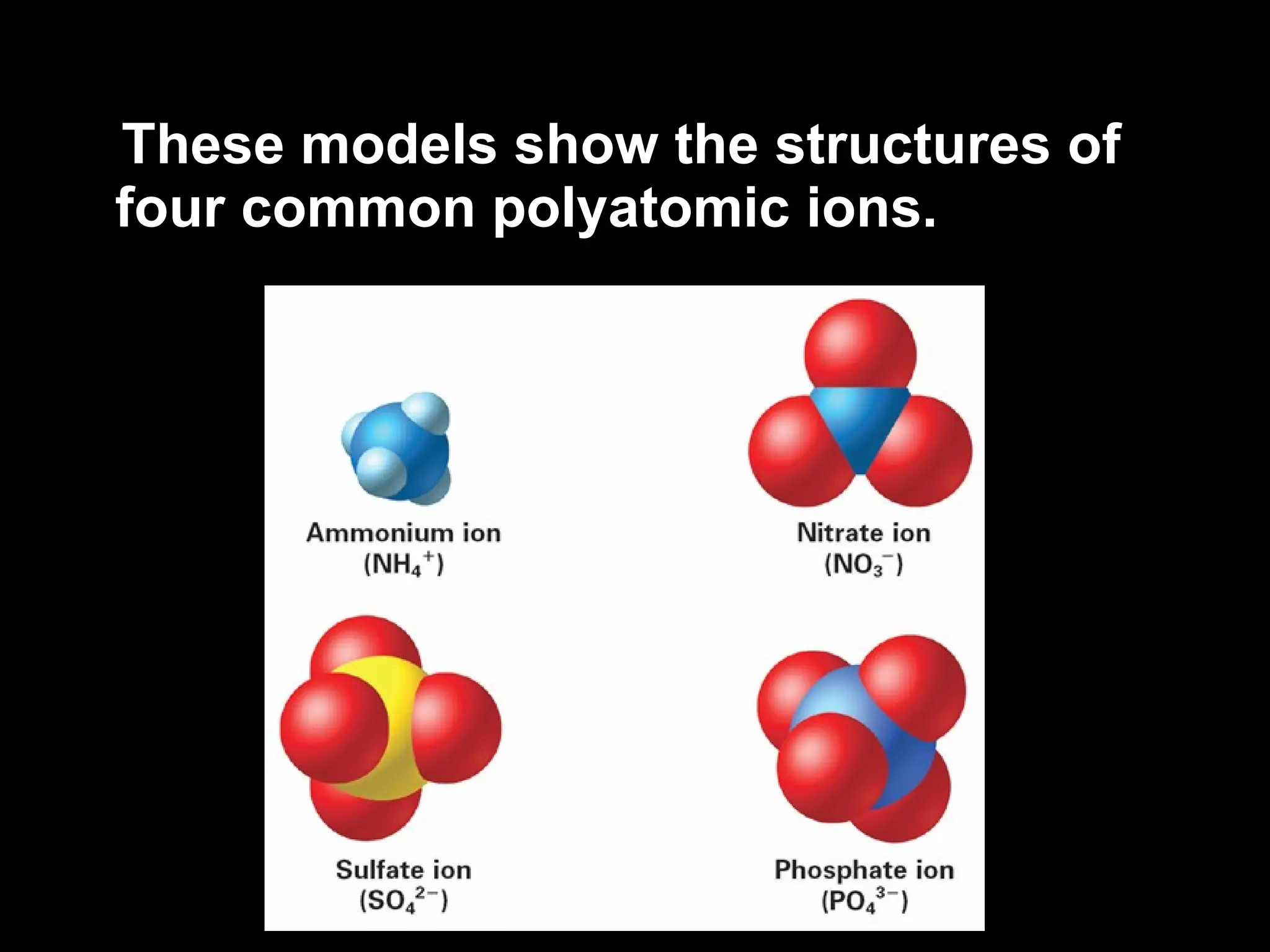
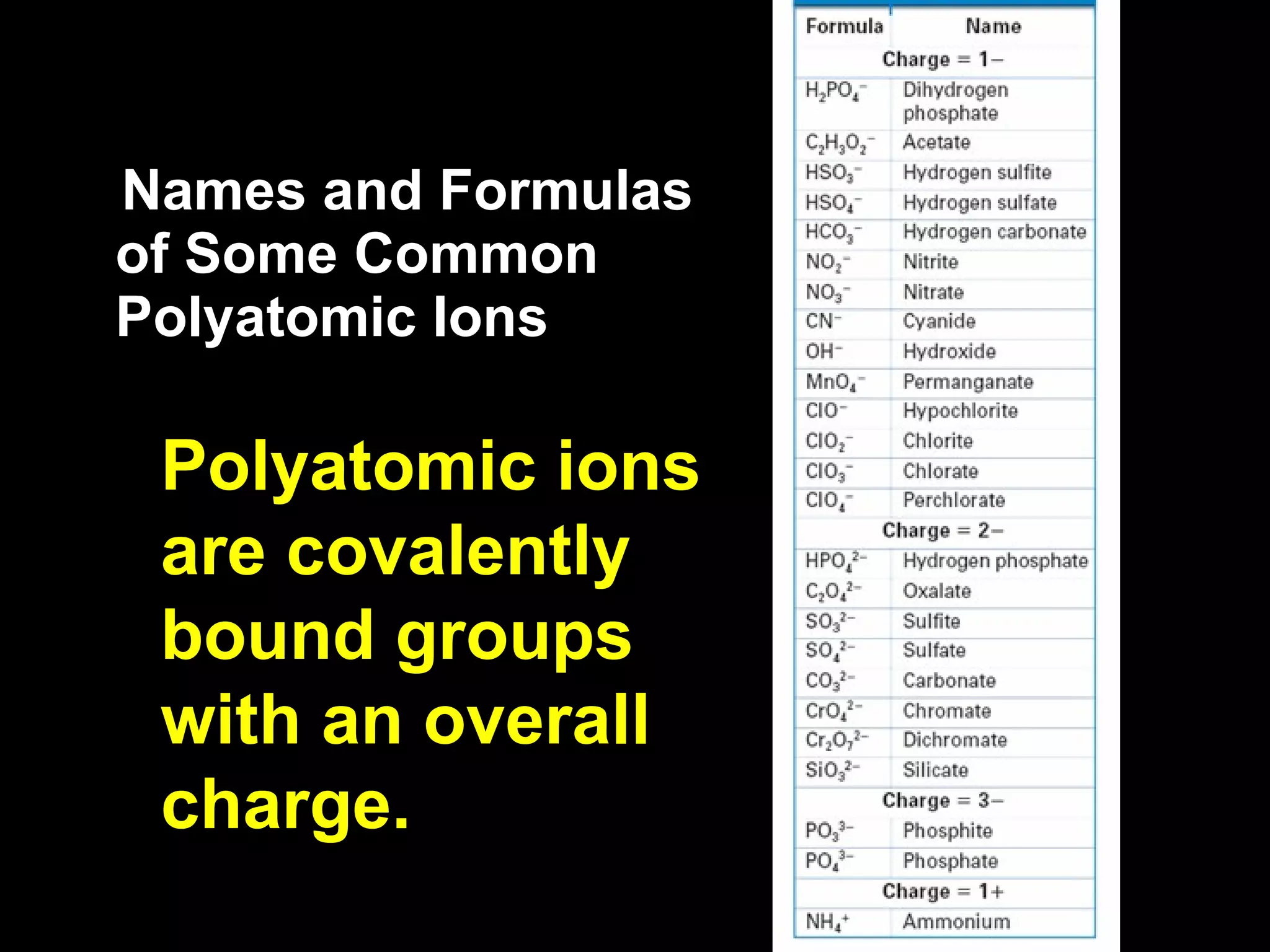
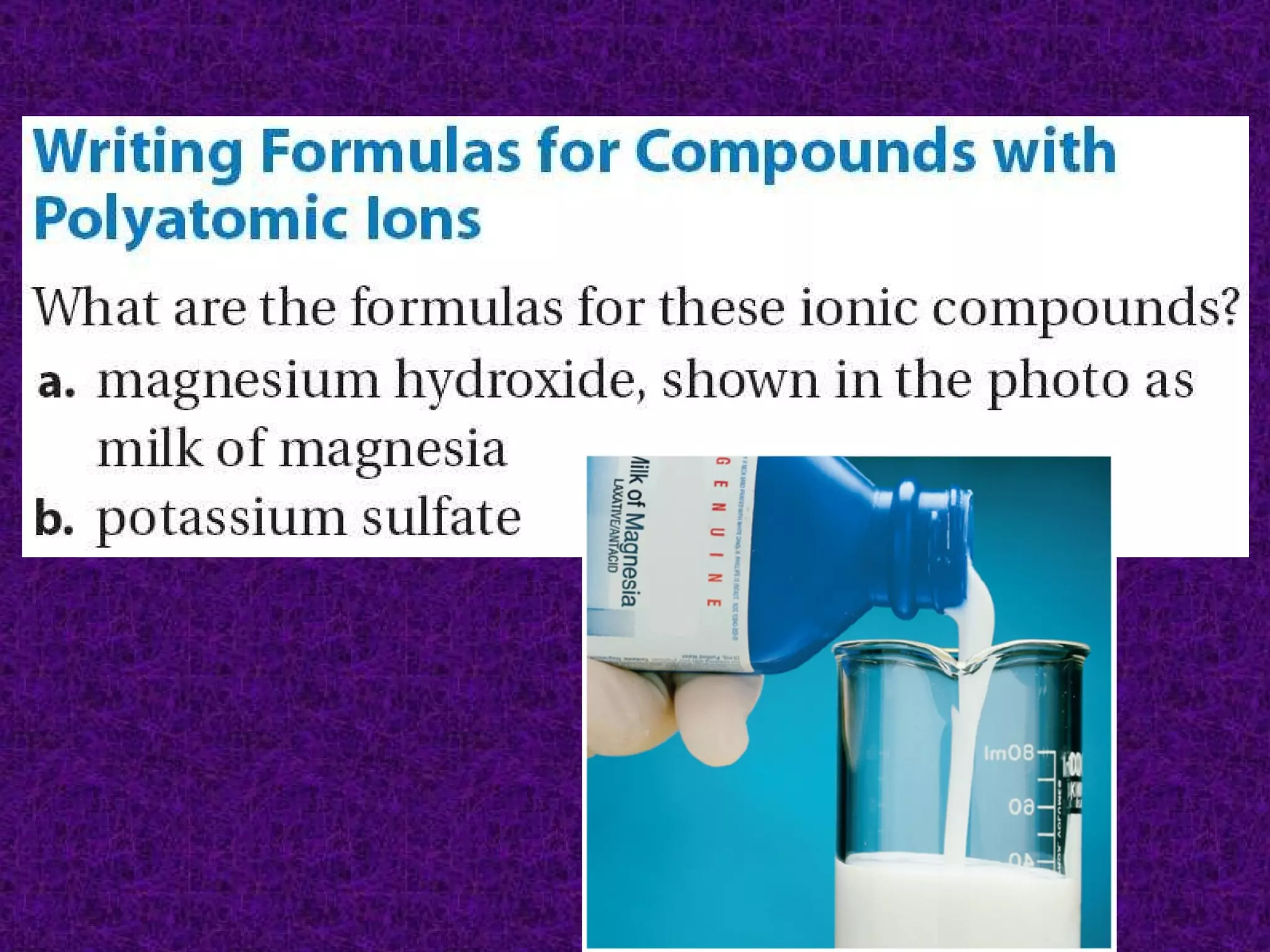
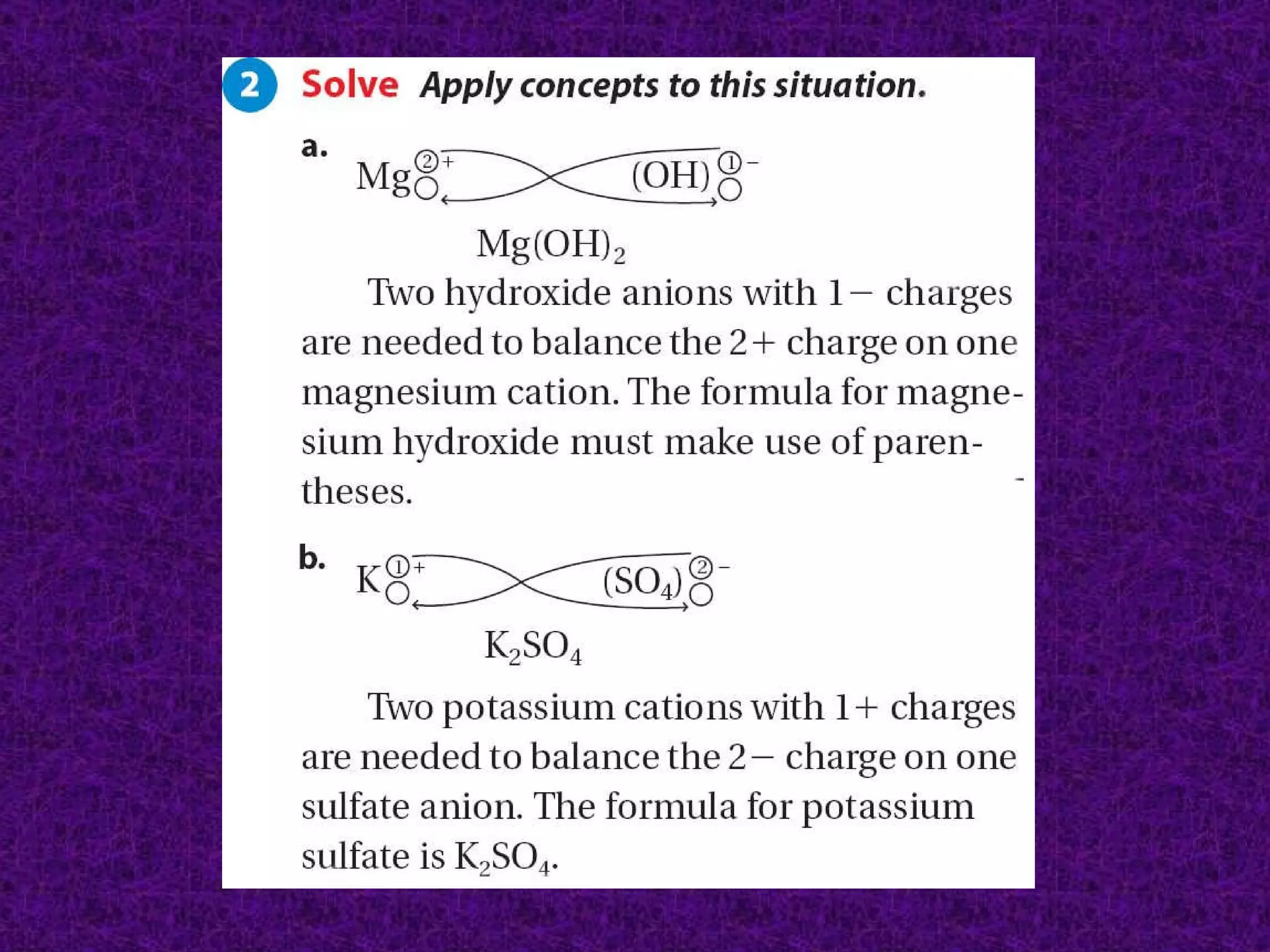
The document discusses chemical names and formulas of ions. It explains that monatomic cations form when metals lose electrons and gain a positive charge equal to their group number. Monatomic anions form when non-metals gain electrons and have a negative charge equal to the group number minus 8. Polyatomic ions consist of multiple atoms and have an overall positive or negative charge. Transition metals can form multiple ions with different charges that must be specified. Ionic compounds are named by writing the cation first followed by the anion.