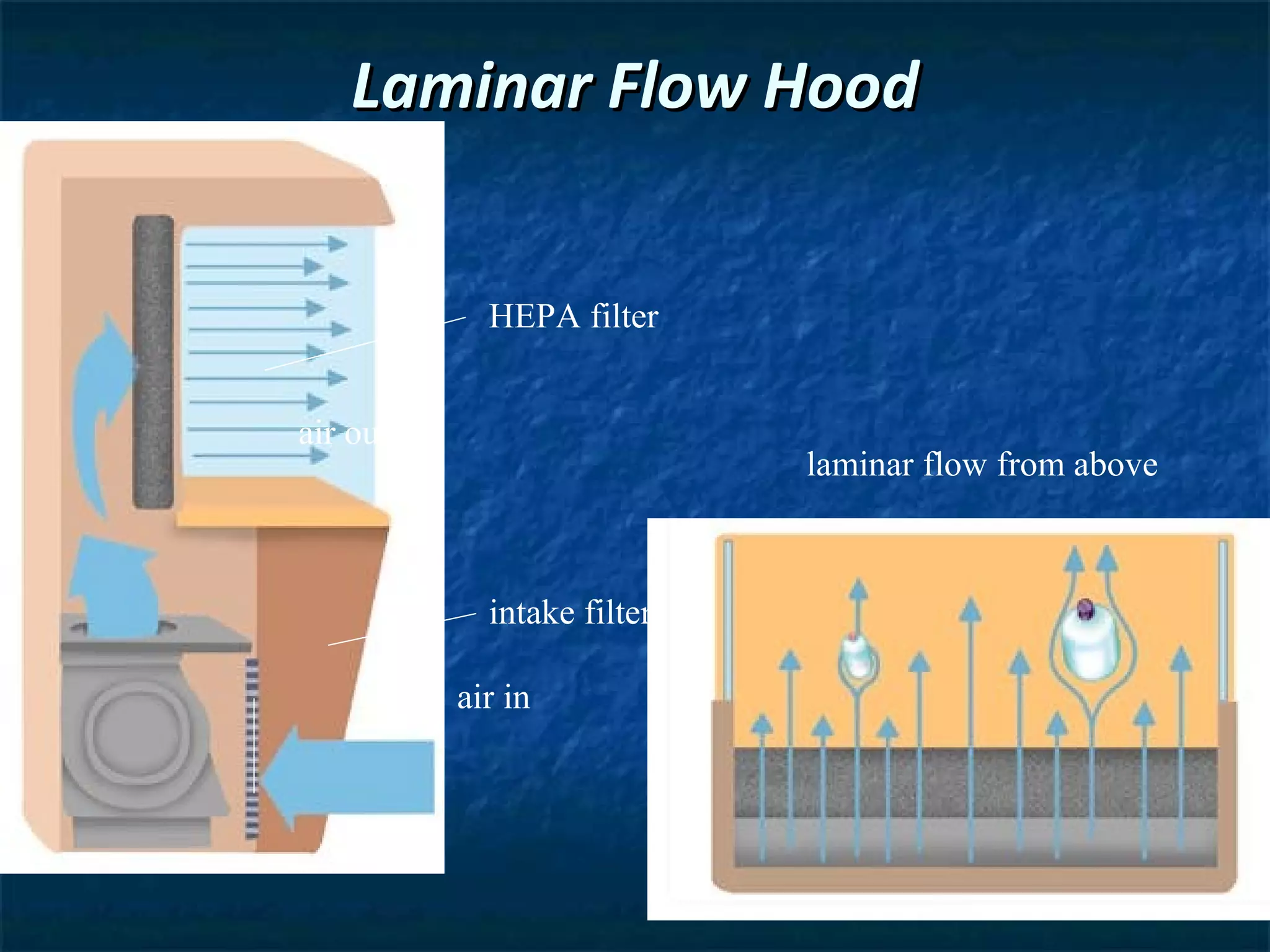
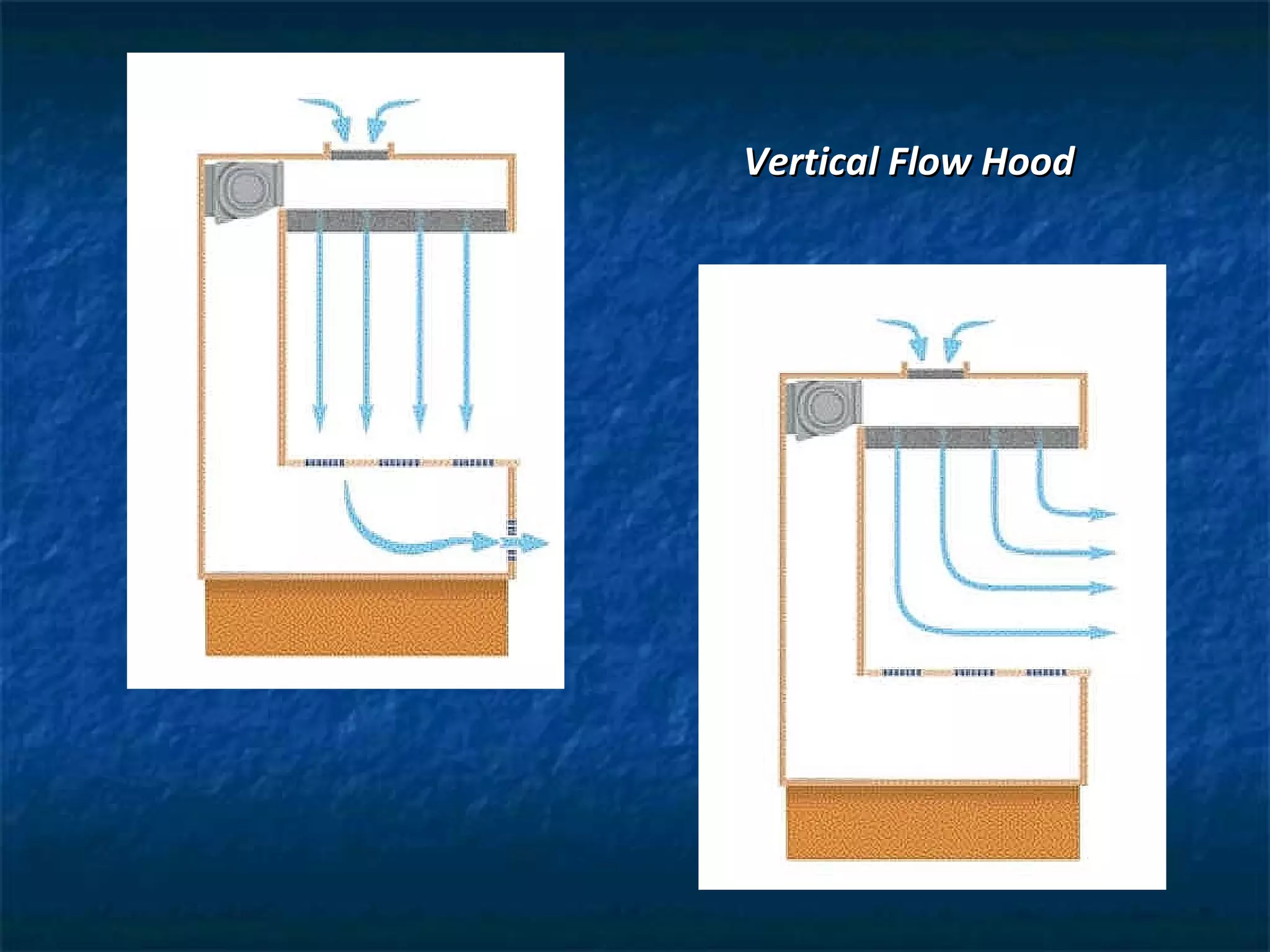
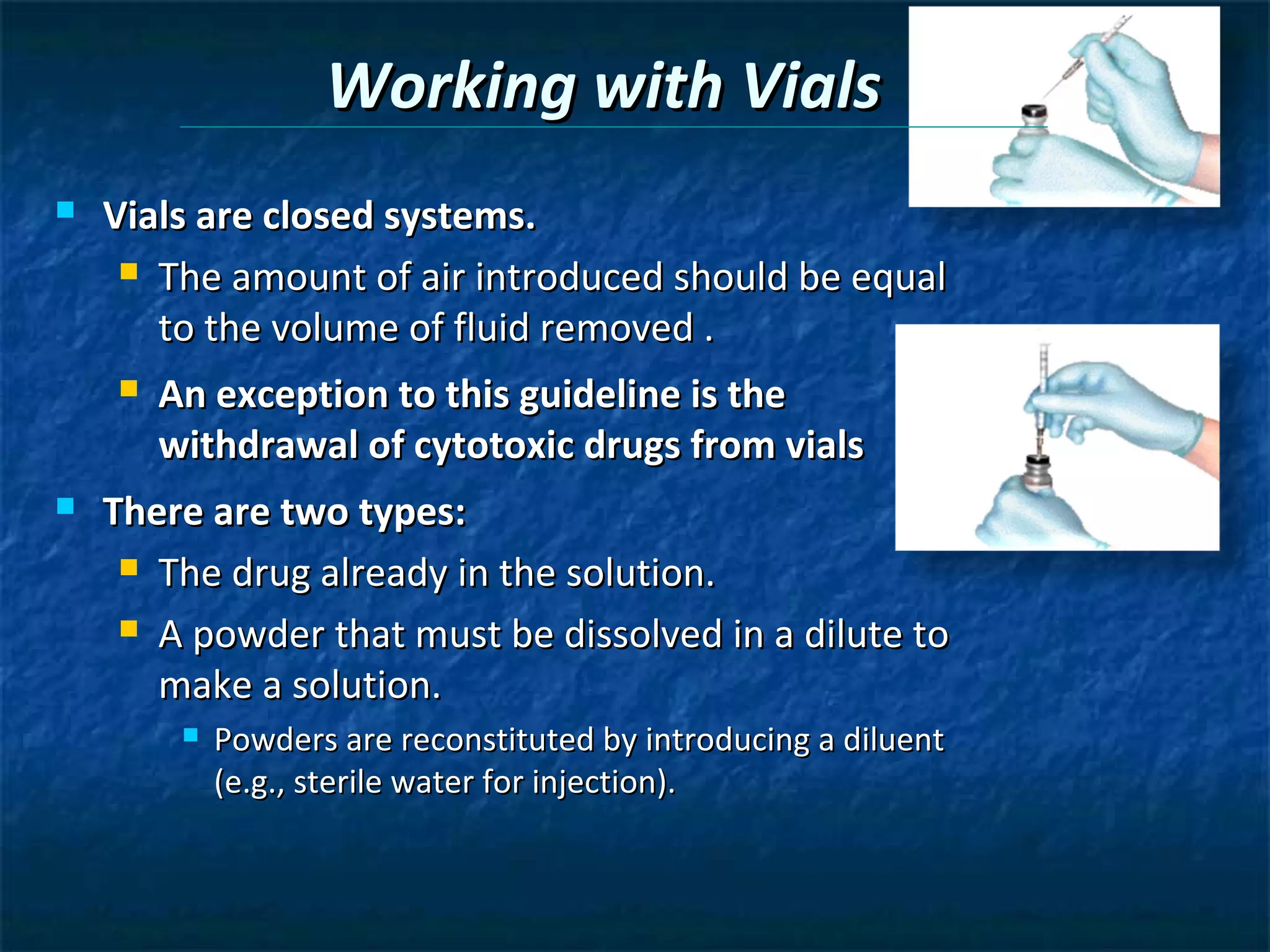
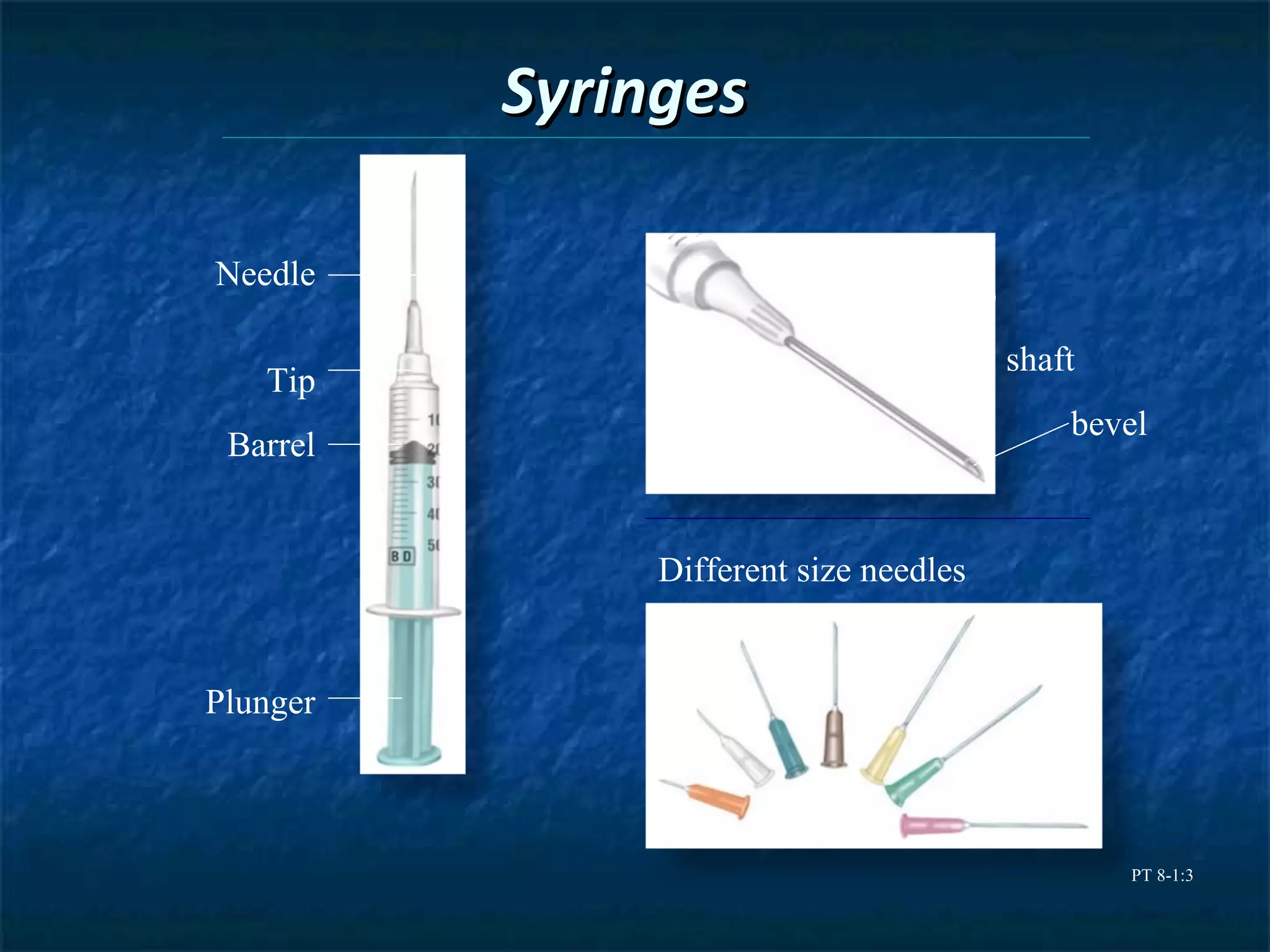
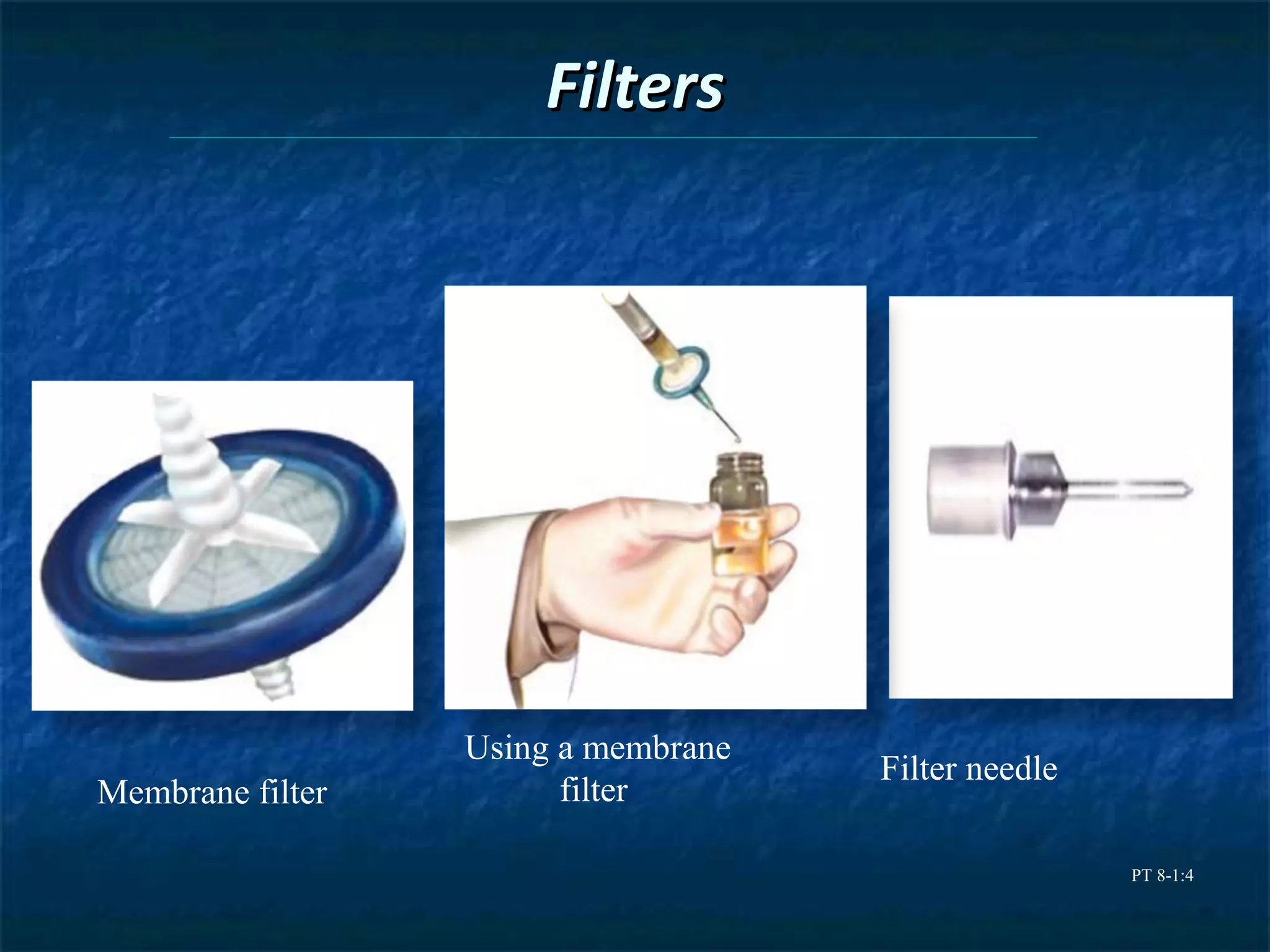
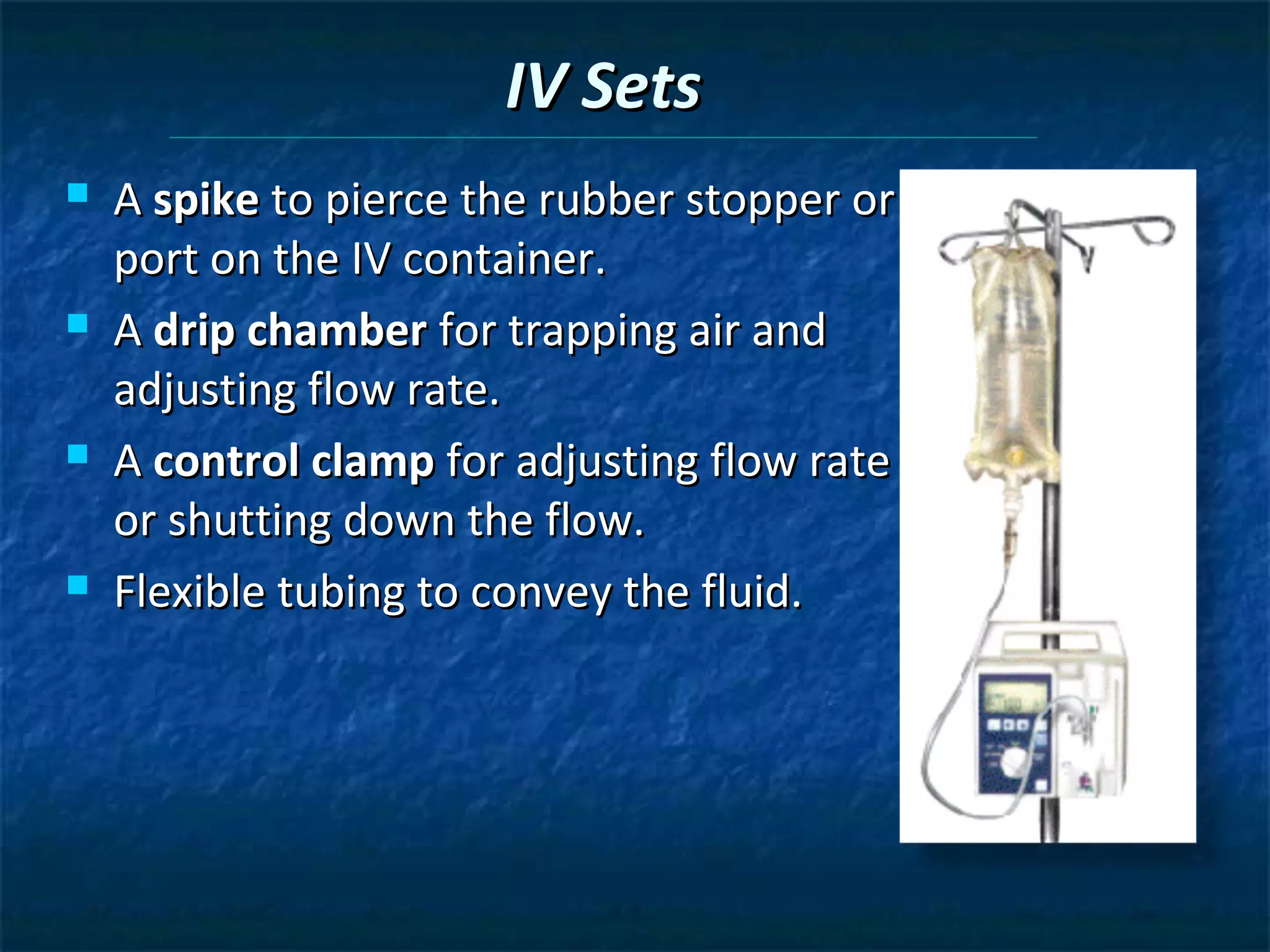
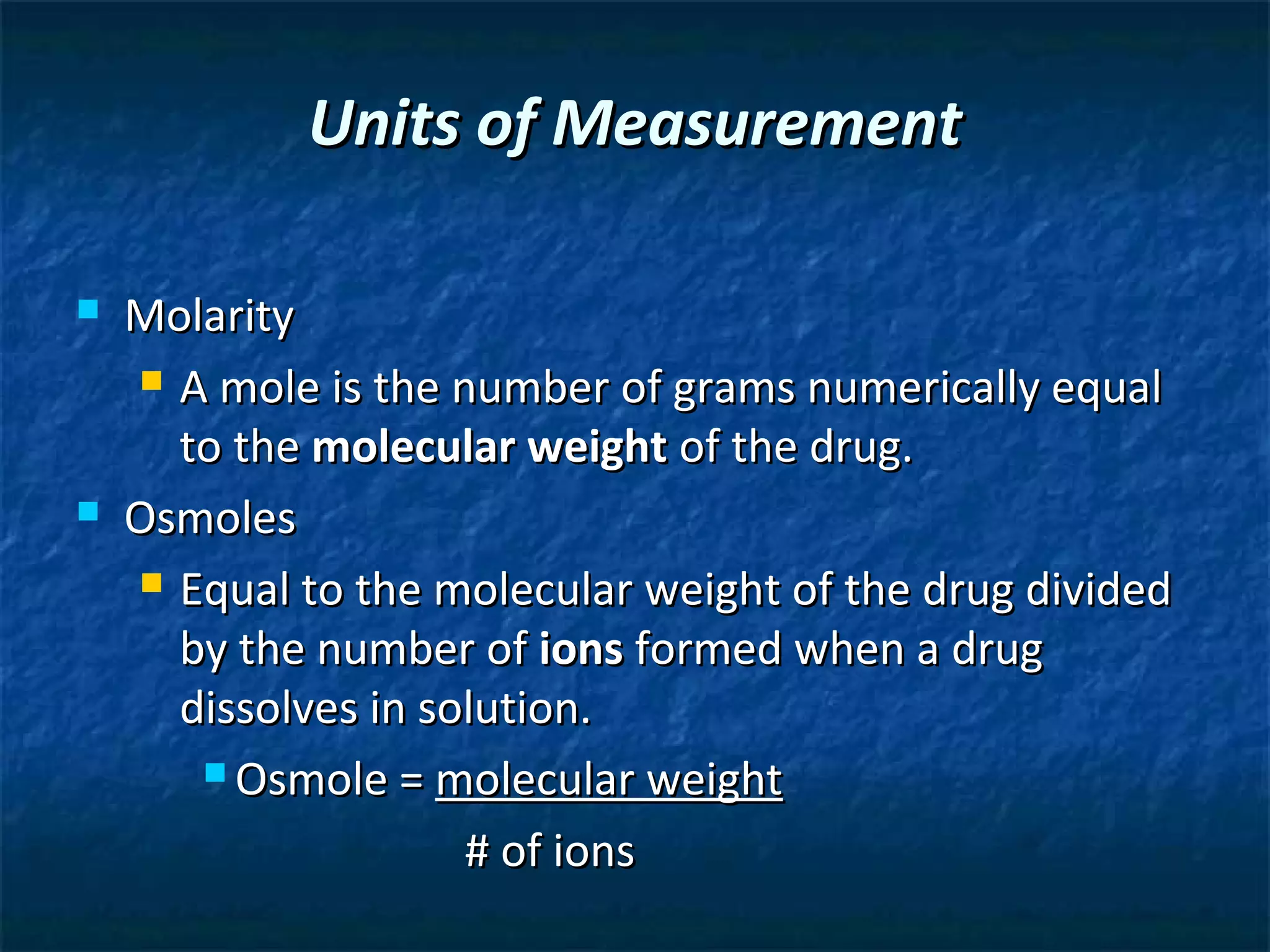
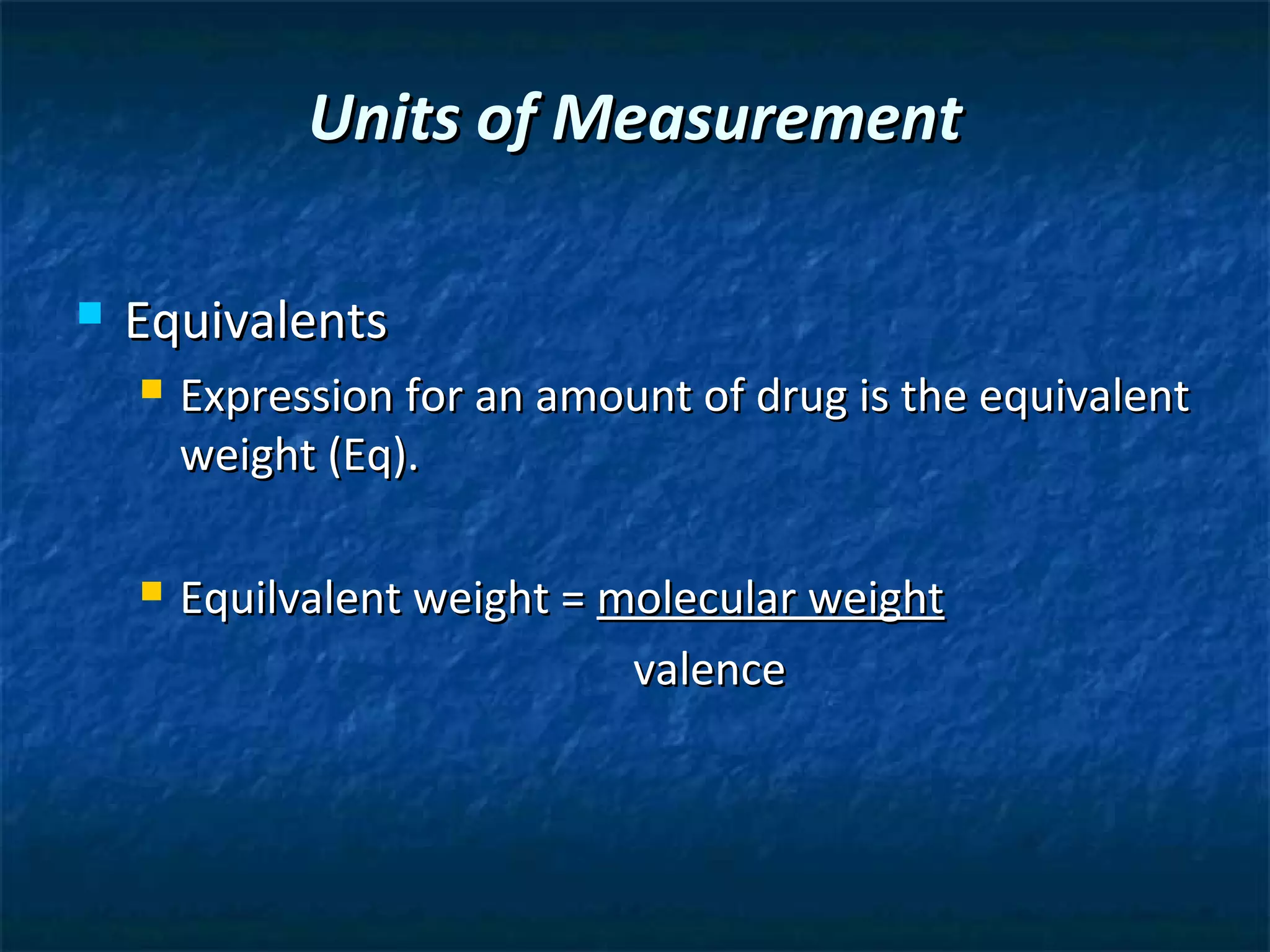
This document discusses preparing sterile formulations, including intravenous (IV) solutions and parenteral drugs. It covers topics such as large volume parenterals (LVPs), small volume parenterals (SVPs), laminar flow hoods, aseptic technique, working with vials and ampoules, and guidelines for preparing IVs. The goal is to prevent contamination and ensure sterility when preparing drugs and solutions that will be administered directly into the bloodstream.