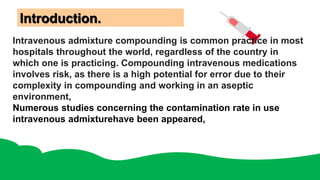
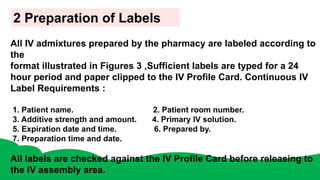
Intravenous admixtures are sterile drug products added to IV fluids for medication purposes. They require strict aseptic technique during preparation to avoid contamination. Compatibility between drugs and solutions must also be considered to prevent precipitation or degradation. While IV admixtures provide benefits like convenience and accurate dosing, they carry higher risks than single drug infusions due to complexity and potential incompatibilities. Proper training and quality control processes are necessary for safe intravenous admixture preparation.