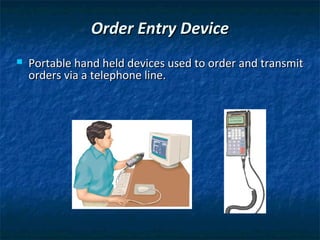
This chapter discusses inventory management in pharmacies. It explains that inventory management ensures medications are available when needed. Various inventory systems track inventory levels and generate reorders. Computer systems automate ordering and inventory tracking. Wholesalers supply medications to pharmacies and handle ordering, shipping, and paperwork. Pharmacies must follow regulations for controlled substances and safe purchasing practices. Proper stocking, storing, and disposal of expired drugs is also covered.