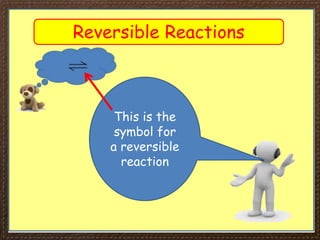
This document discusses reversible reactions and how they relate to closed systems. It introduces the concept of reversible reactions which can proceed in both directions, using examples like the heating and cooling of copper sulfate. It then explains that a closed system at dynamic equilibrium occurs when the forward and backward reaction rates are equal. Finally, it describes how temperature and pressure can impact the equilibrium position - increasing temperature favors the endothermic direction, while increasing pressure favors the side with fewer moles of gas.