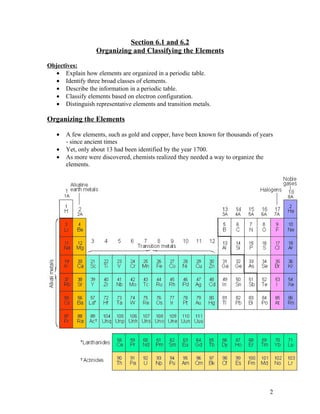
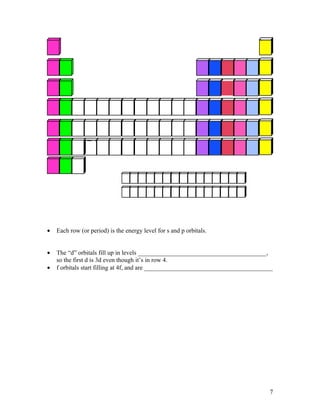
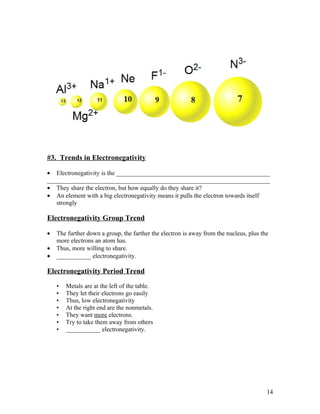
The document summarizes key concepts about organizing elements in the periodic table and periodic trends. It explains that elements are organized based on their atomic number and electron configuration into groups with similar properties. Periodic trends are discussed, including how atomic size decreases left to right across a period but increases down a group, and how ionization energy and electronegativity increase left to right and decrease down a group. Cations are smaller than their parent atoms while anions are larger.