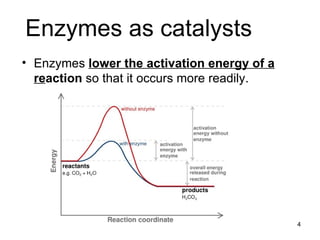
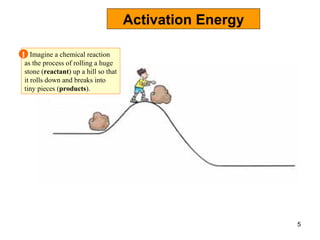
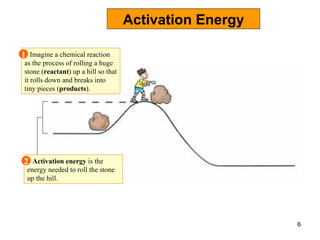
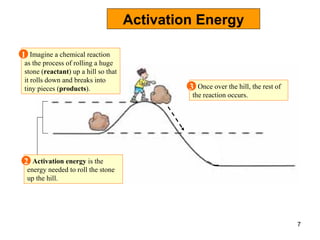
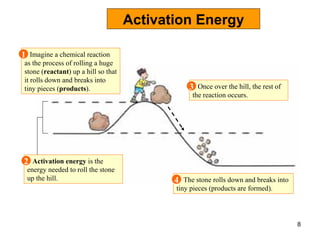
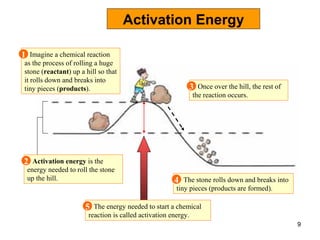
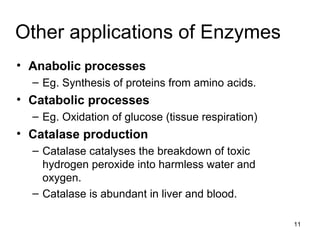
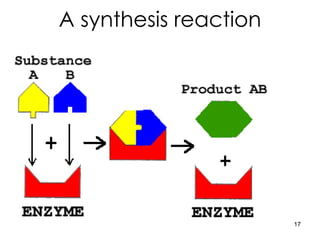
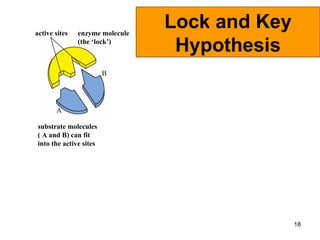
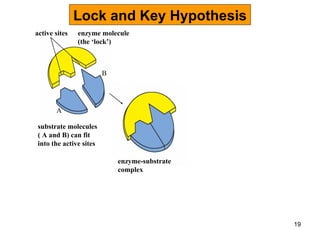
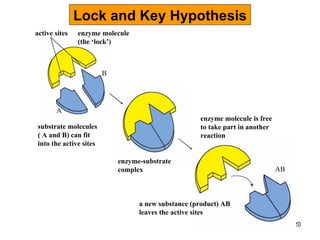
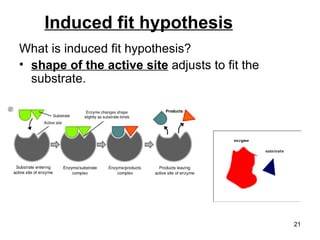
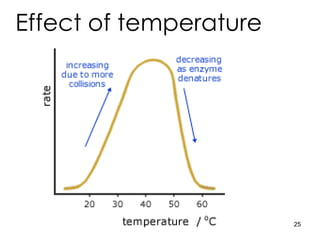
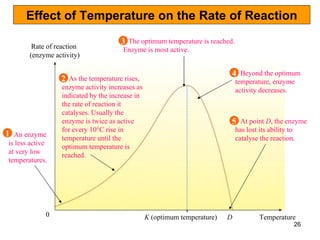
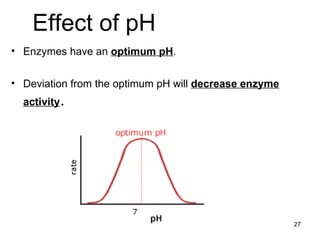
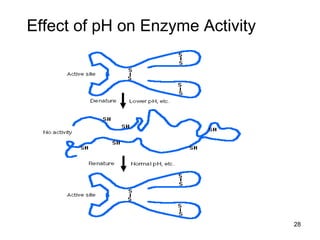
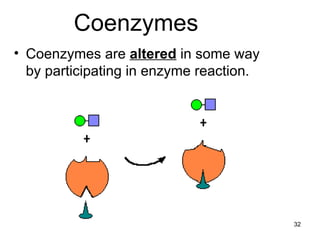
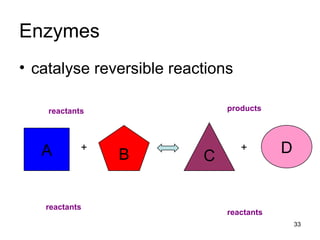
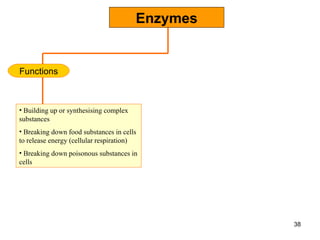
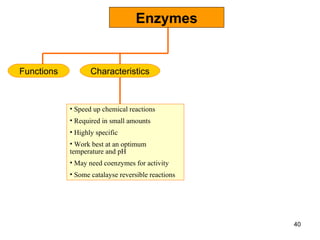
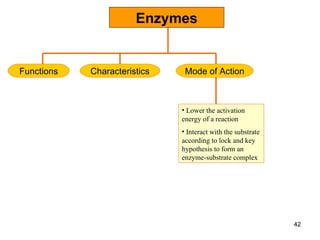
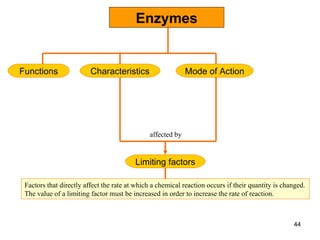
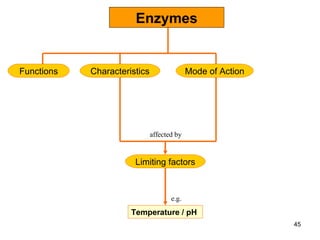
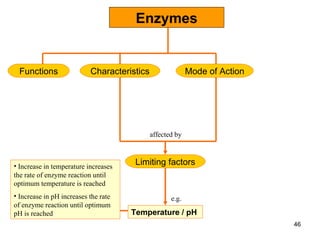
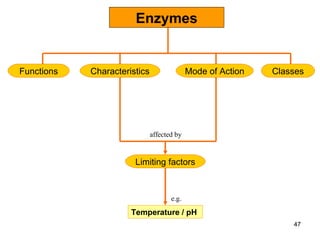
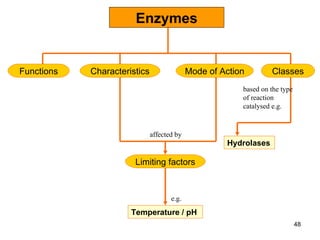
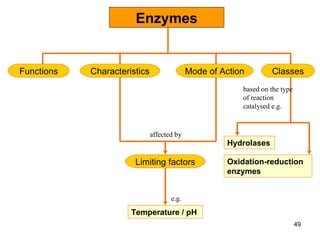
Enzymes are proteins that catalyze chemical reactions without being altered themselves. They speed up reactions by lowering activation energy. Enzymes work most efficiently at a specific temperature and pH, and require cofactors to function. Enzyme activity is affected by substrate, temperature, pH, and other limiting factors. Enzymes are classified based on the type of reaction they catalyze, such as hydrolysis or oxidation-reduction.