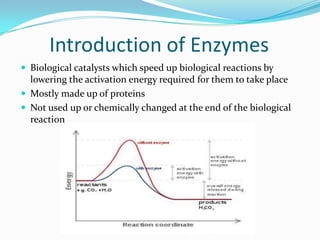
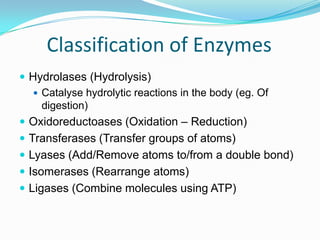
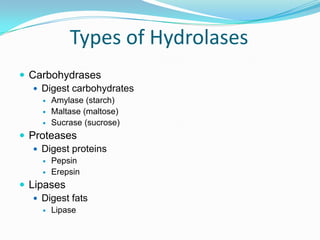
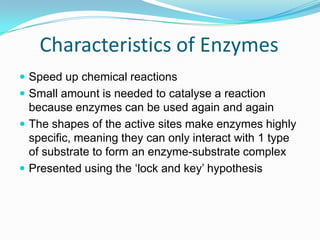
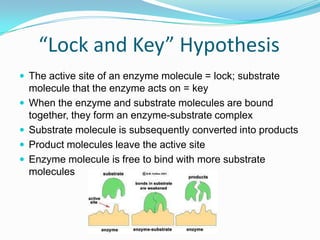
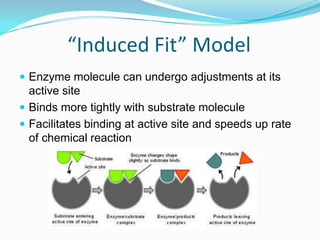
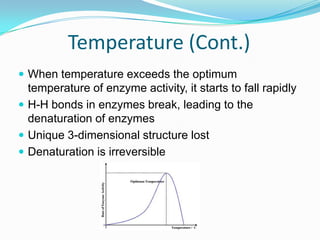
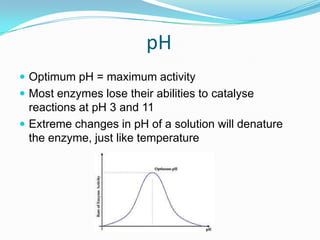
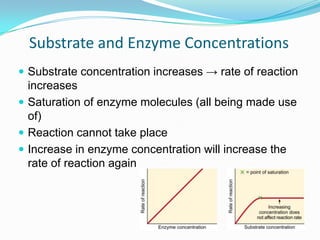
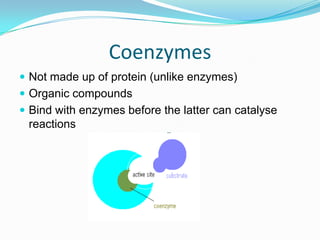
Enzymes are biological catalysts that speed up biochemical reactions by lowering their activation energy. They are mostly proteins and are not used up in the reactions they catalyze. Enzymes can catalyze both anabolic reactions that build up complex molecules and catabolic reactions that break down complex molecules. They are classified based on the type of reaction they catalyze such as hydrolases, oxidoreductases, transferases, lyases, isomerases, and ligases. Enzyme activity is affected by factors like temperature, pH, and substrate and enzyme concentrations, with most enzymes having an optimal temperature and pH at which their activity is highest before they become irreversibly denatured.