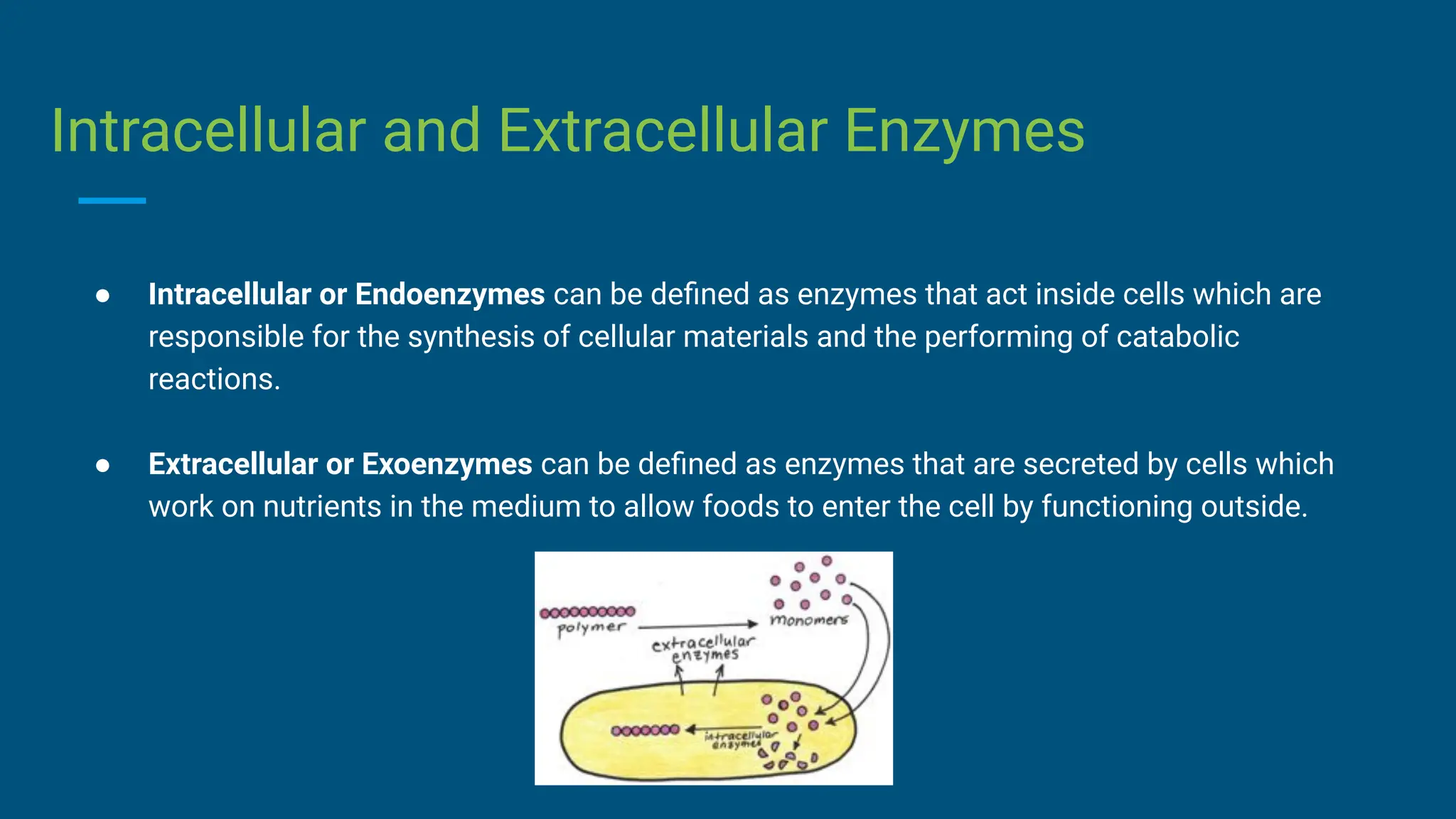
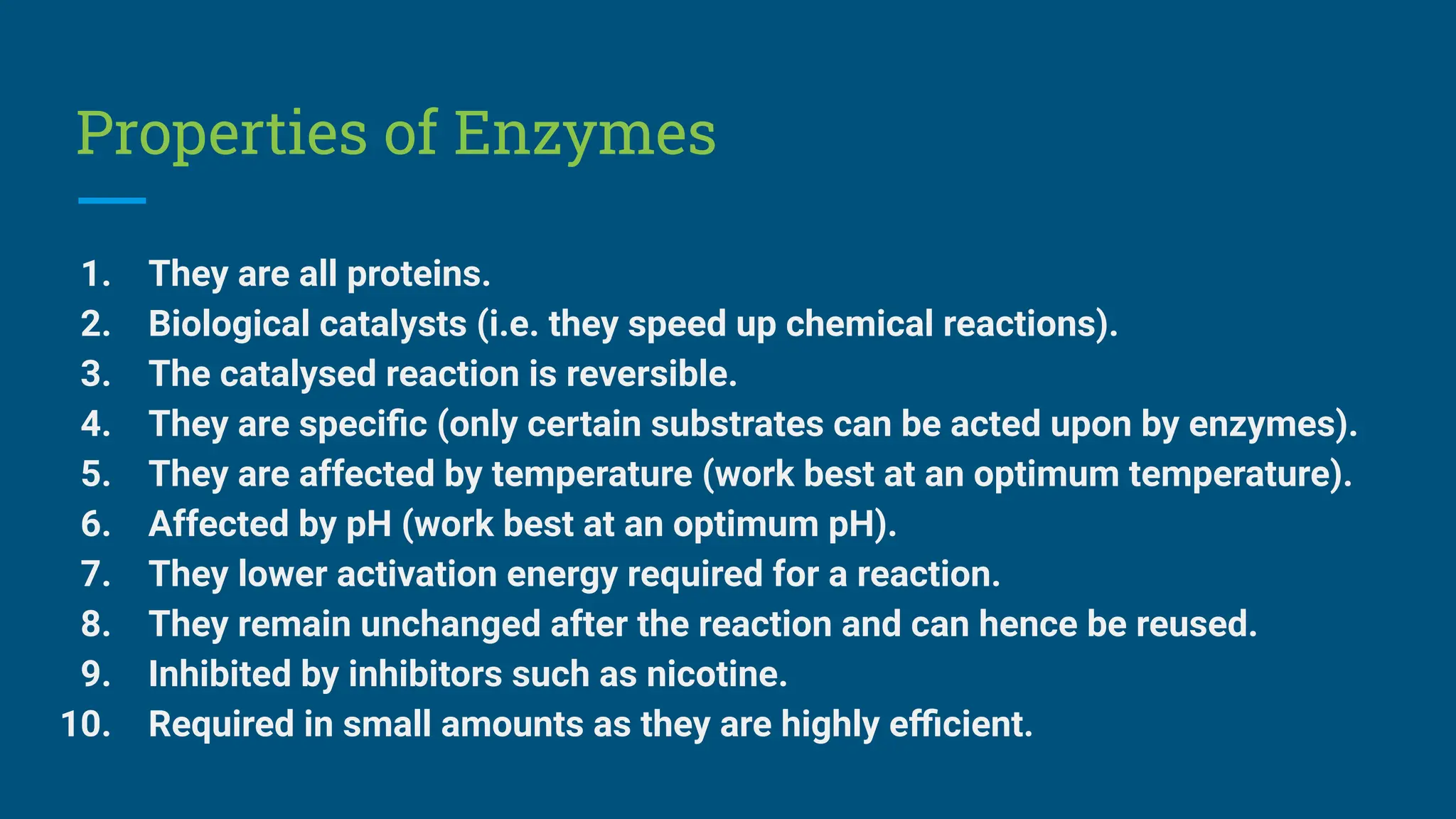
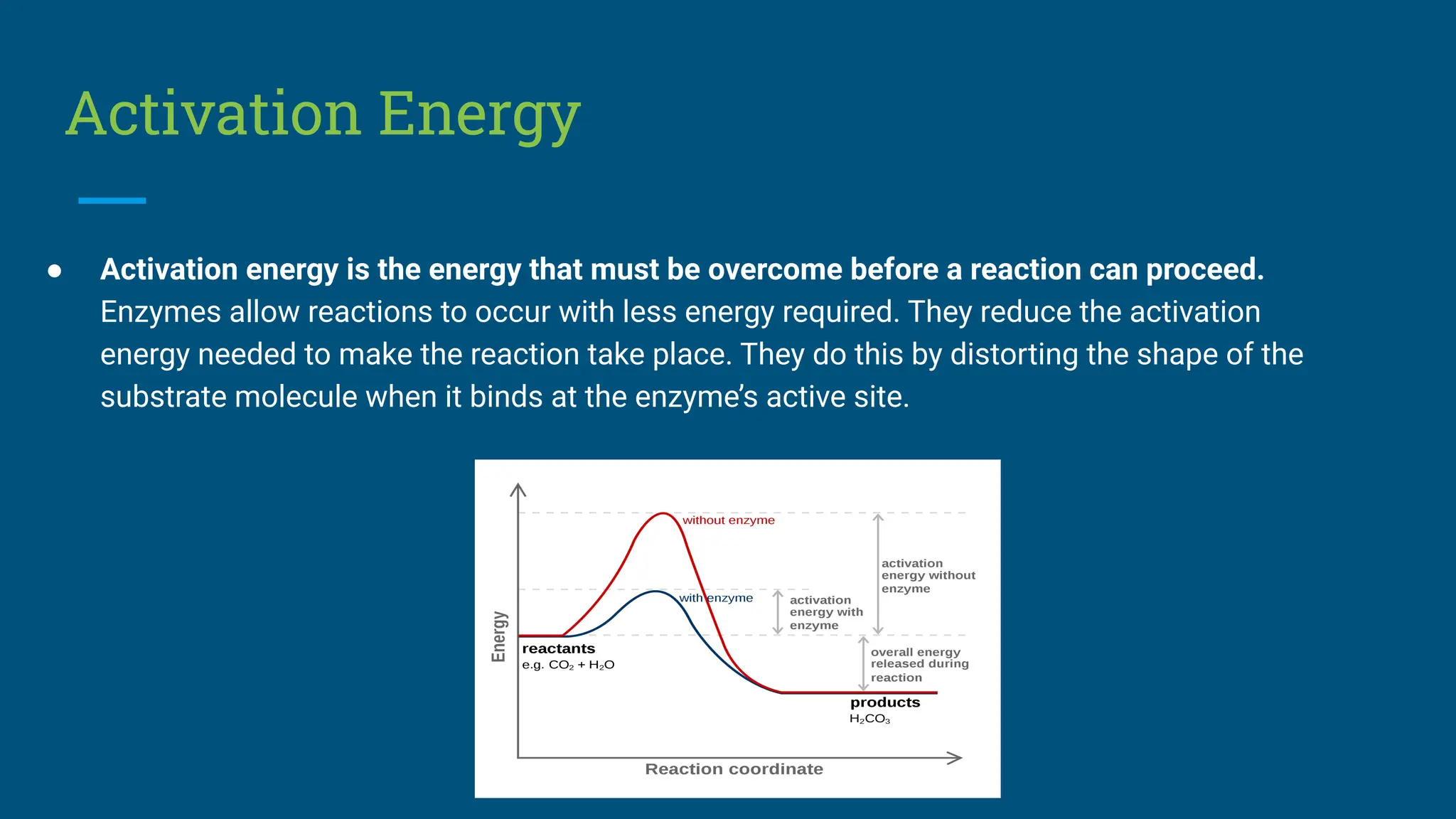
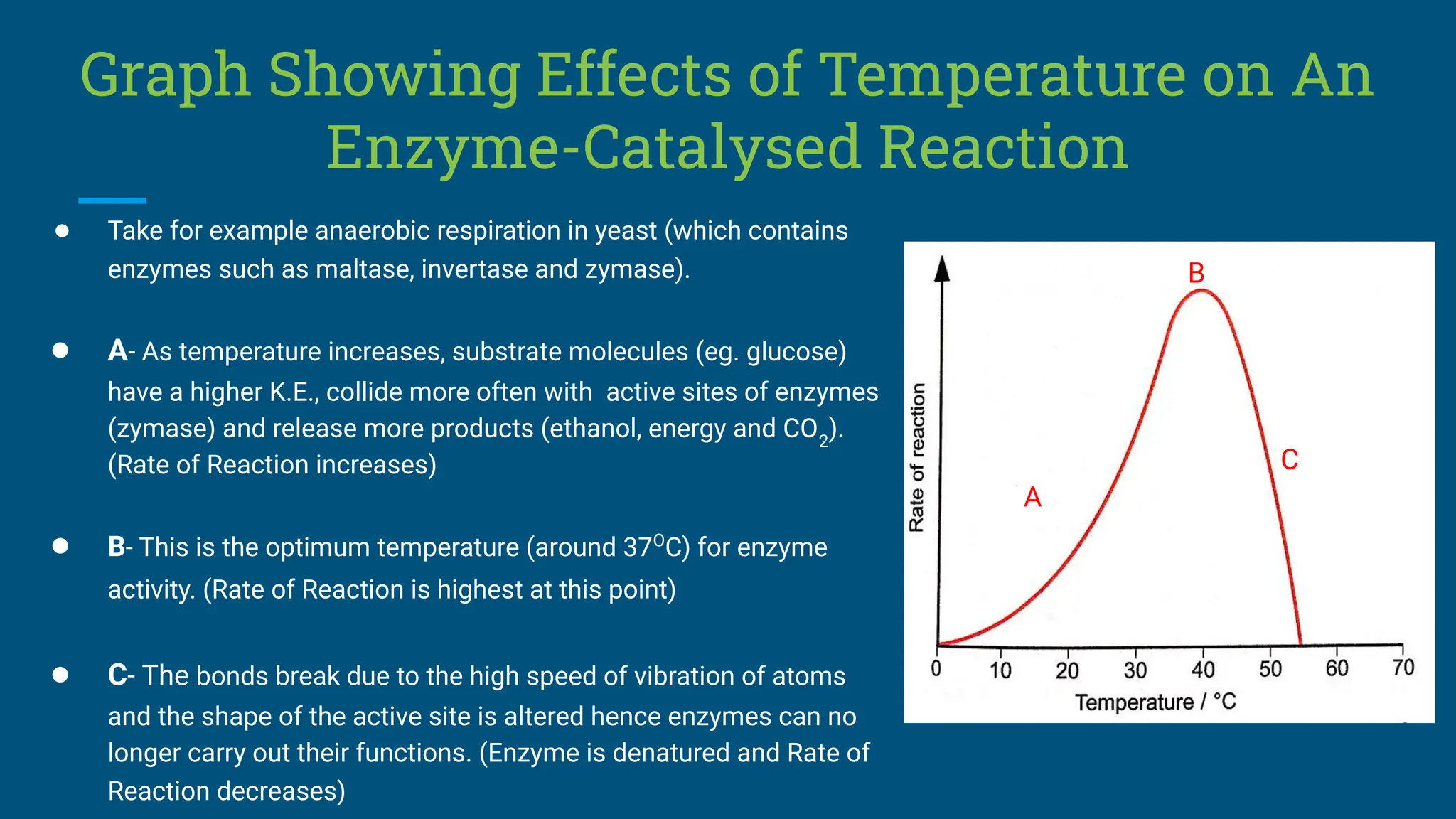
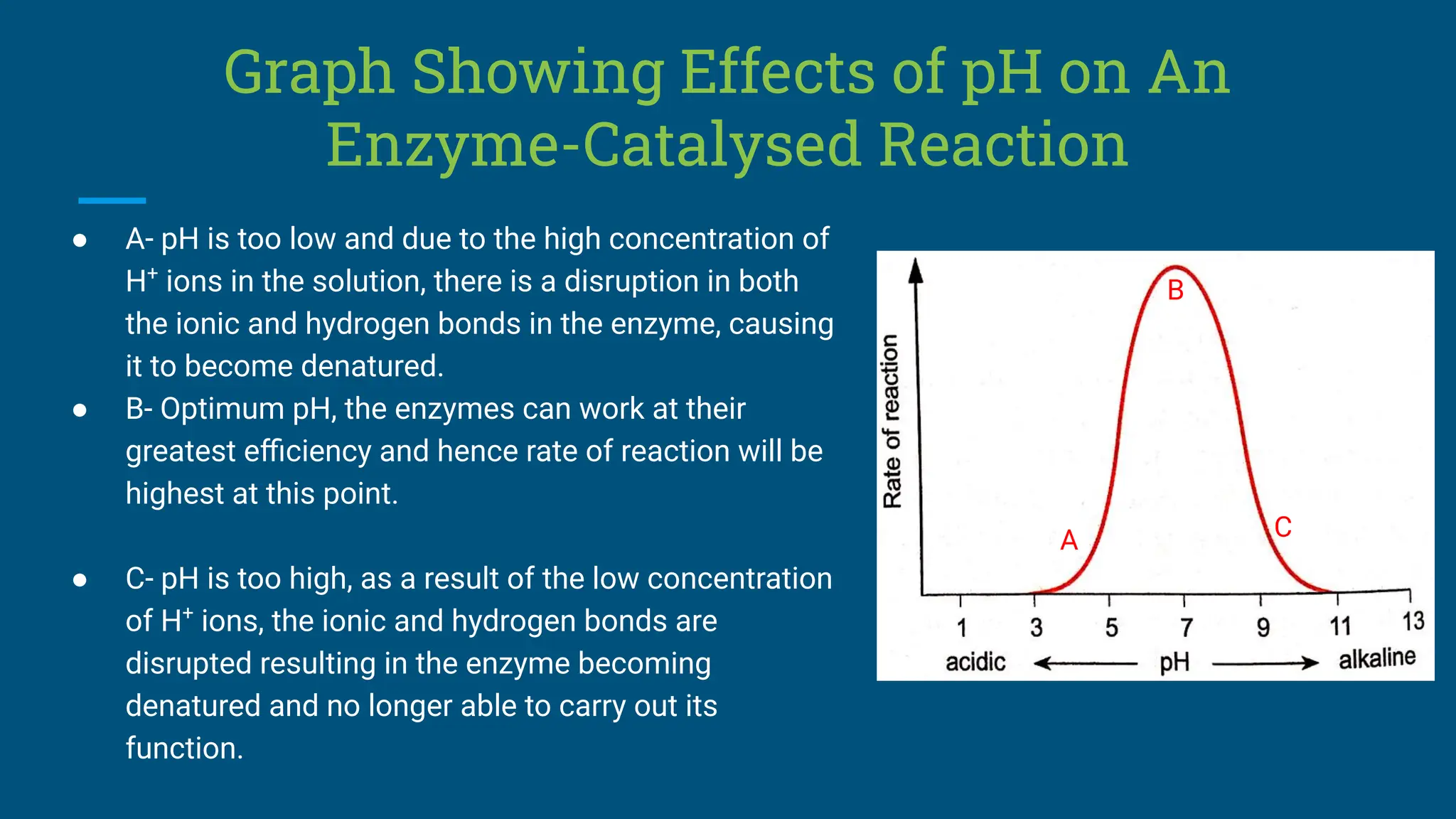
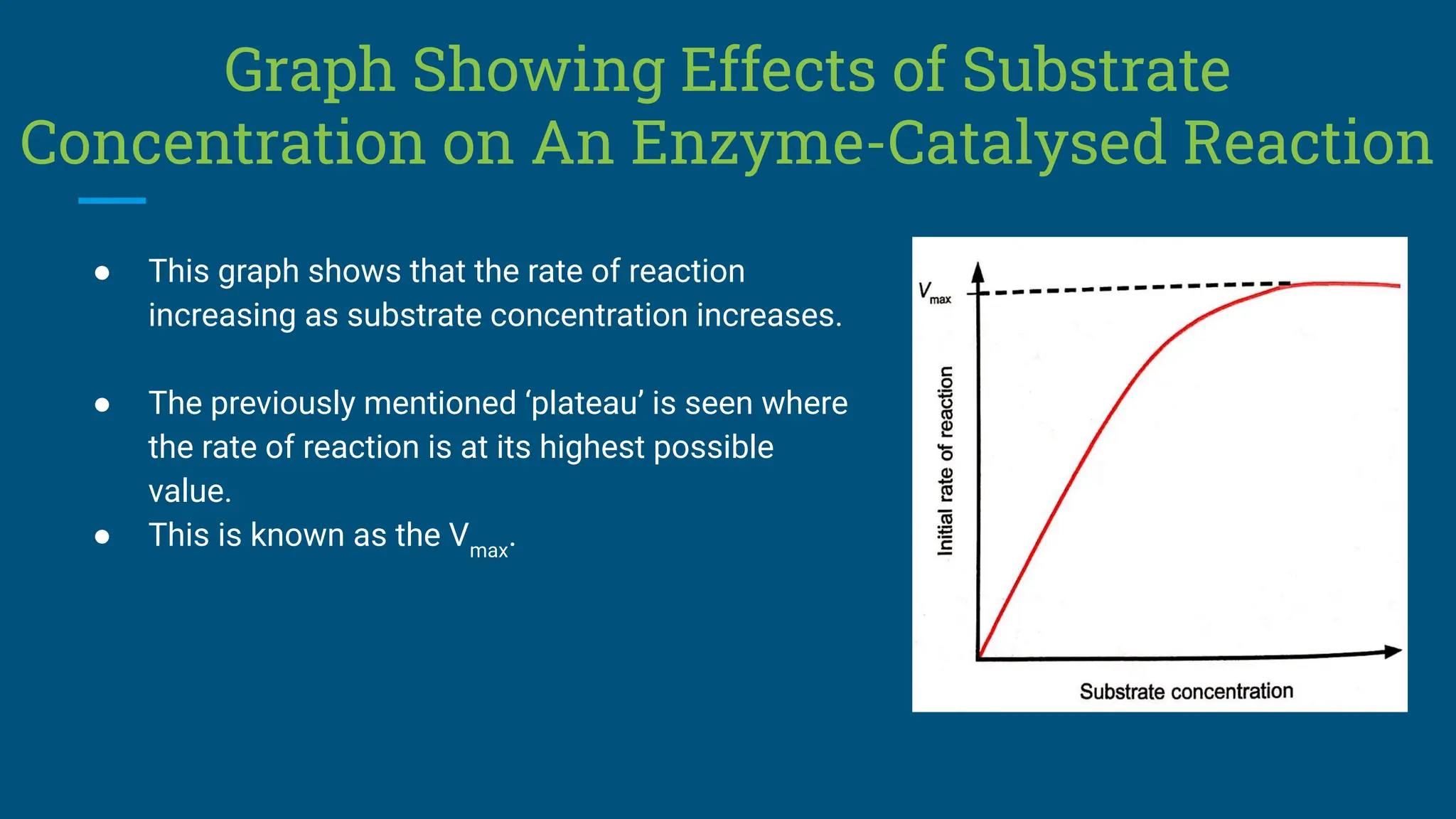
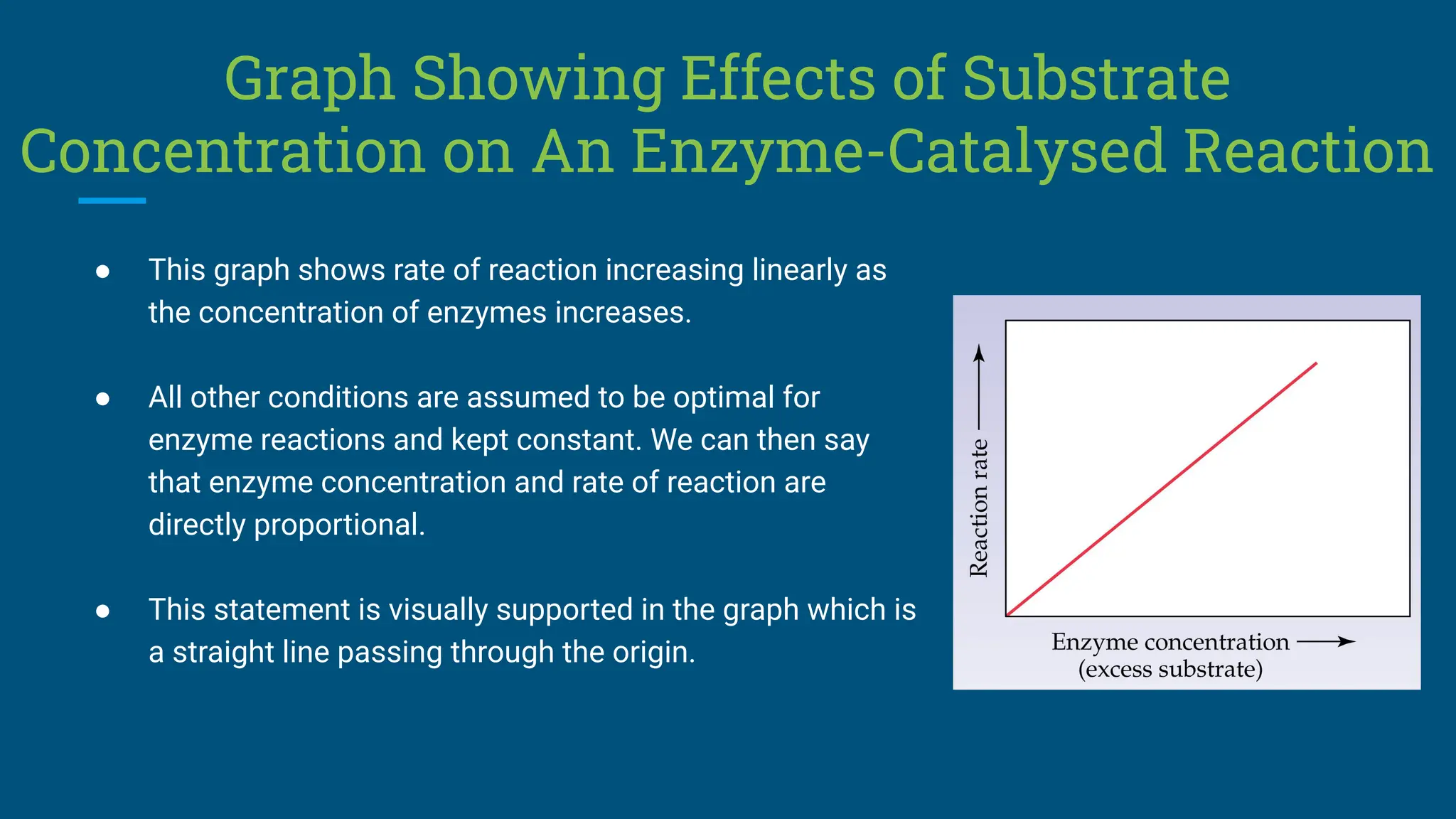
The document covers the definition, types, and properties of enzymes, which are biological catalysts that speed up metabolic reactions without being changed themselves. It details the mechanisms of enzyme action, including the active site, enzyme-substrate complex, and the differences between lock and key and induced fit hypotheses, as well as factors affecting enzyme activity such as temperature, pH, and substrate concentration. Additionally, it discusses the impact of inhibitors on enzyme function, including competitive and non-competitive inhibition.