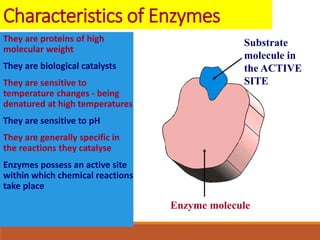
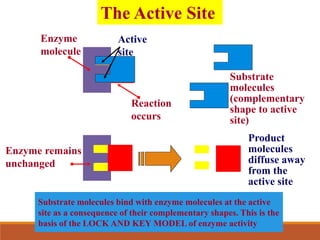
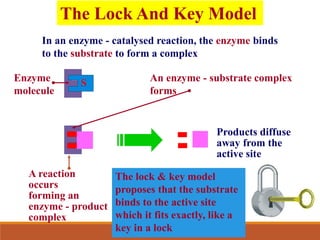
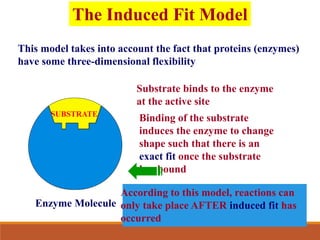
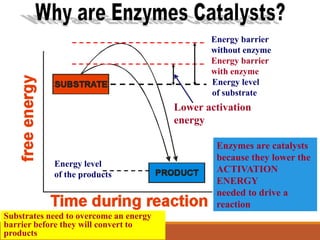
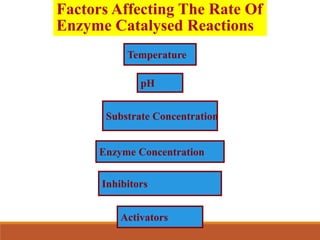
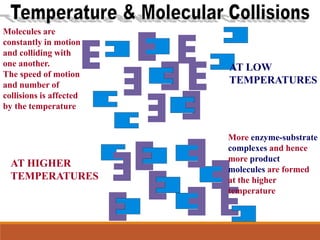
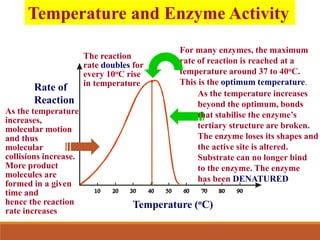
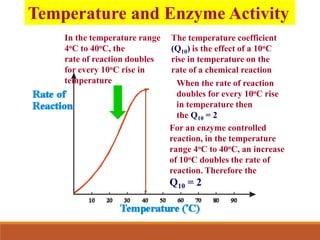
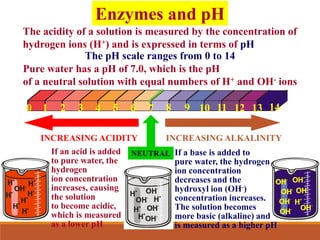
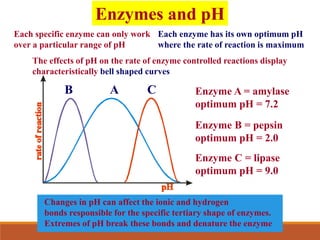
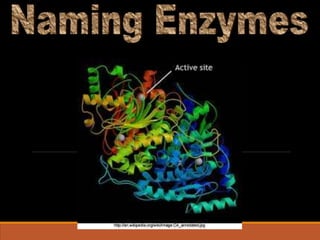
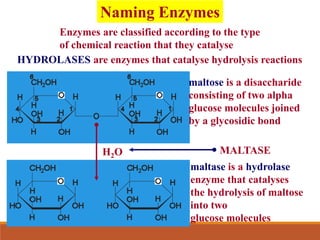
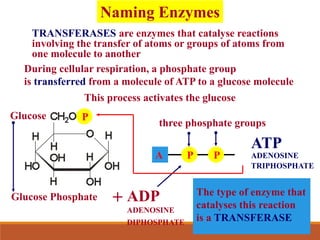
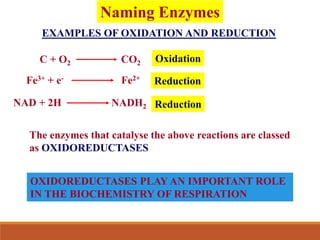
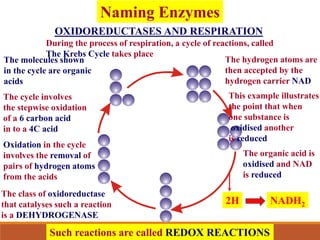
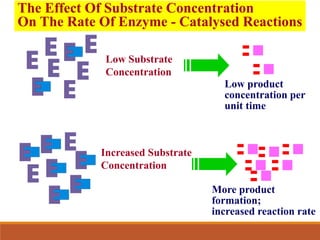
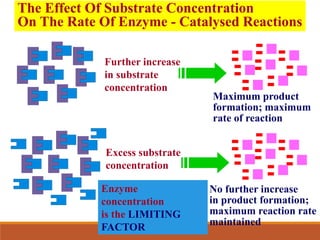
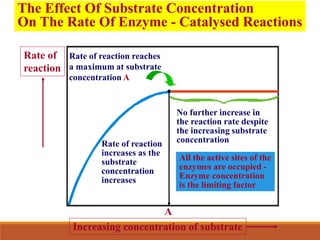
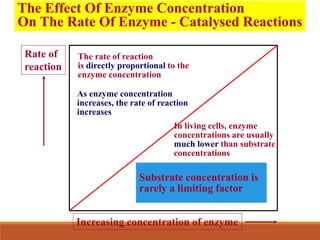
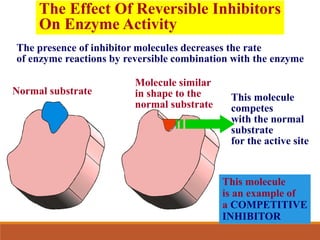
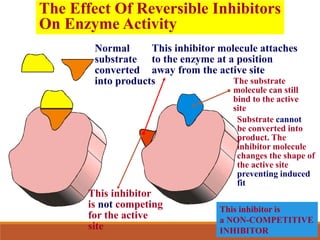
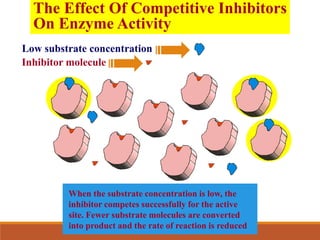
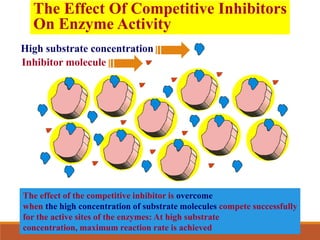
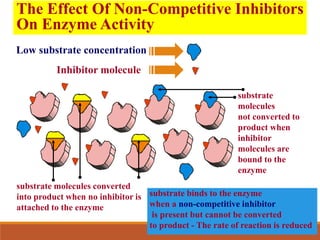
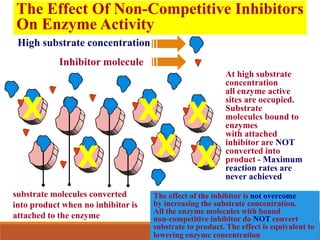
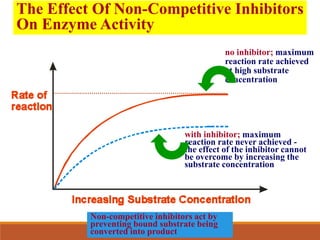
Enzymes are protein catalysts that greatly increase the rate of biochemical reactions. They work by lowering the activation energy of reactions. The active site of an enzyme complements the substrate and catalyzes its conversion to products. Factors like temperature, pH, substrate and enzyme concentration can affect the rate of enzyme-catalyzed reactions. Inhibitors bind to enzymes and decrease reaction rates, with competitive inhibitors binding the active site and non-competitive inhibitors binding elsewhere.