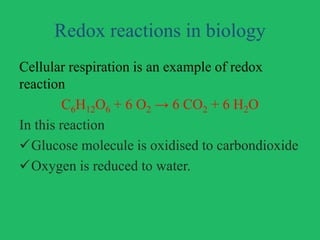
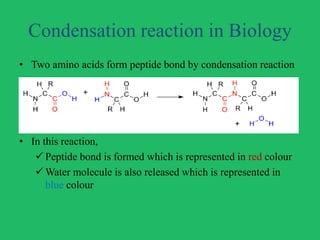
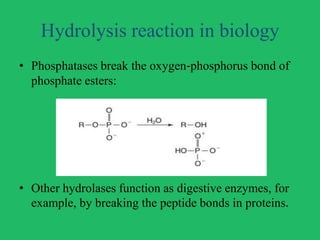
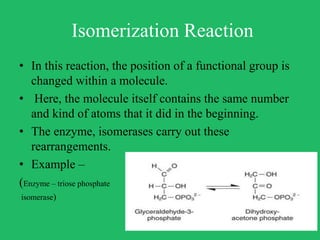
The document outlines vital biological processes and the types of chemical reactions involved, focusing on metabolism, which encompasses all biochemical reactions in living organisms. It explains various biochemical reactions such as neutralization, oxidation-reduction, condensation, hydrolysis, group transfer, and isomerization, while highlighting the role of enzymes as catalysts that facilitate these reactions. The importance of maintaining pH levels and how metabolic pathways orchestrate life-sustaining processes in the body is also discussed.