The document provides an overview of key concepts in electrochemistry including:
1) The components and operation of electrochemical cells including voltaic cells like batteries and fuel cells as well as electrolytic cells.
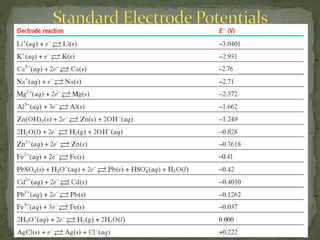
2) Half-reactions, electrode potentials, and using these to determine spontaneity of redox reactions.
3) Processes like corrosion, electroplating, electrolysis of water, and recharging of batteries that involve redox reactions driven by electrical energy.