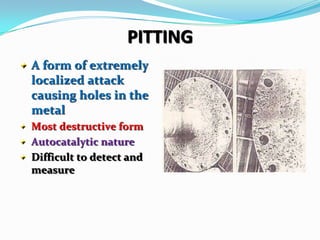
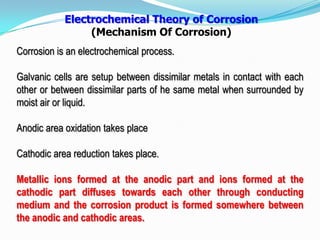
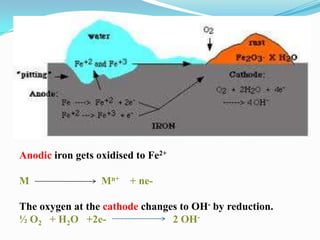
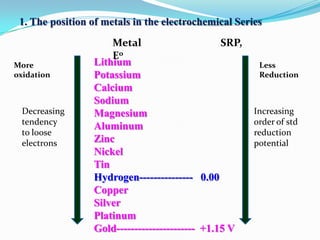
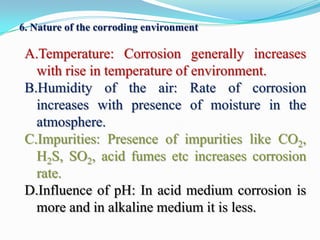
Group 6 presents information on corrosion, including definitions, types of corrosion, factors that affect corrosion rates, and methods for preventing corrosion. The group members are Waqas Ahmad, Umair Aslam, Tayyab Naveed, Muhammad Umair, and Muhammad Mudeser khalid. Corrosion is defined as the decay of metal due to chemical reactions with gases in the atmosphere. There are two main types of corrosion: dry corrosion caused by direct chemical reactions, and wet corrosion which is an electrochemical process. Factors that influence corrosion rates include the metal's position in the galvanic series, purity, physical state, and properties of the corrodent environment. Prevention methods consist of modifying