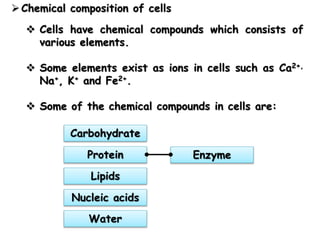
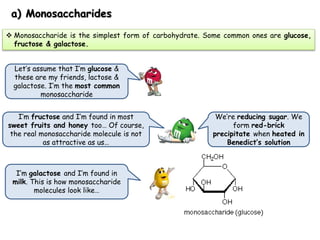
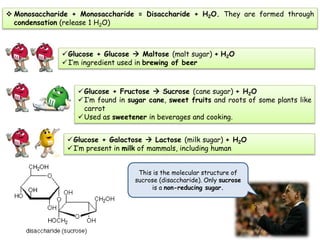
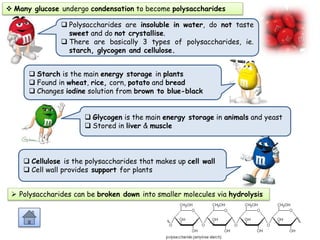
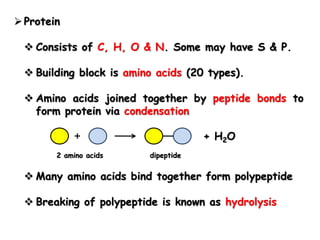
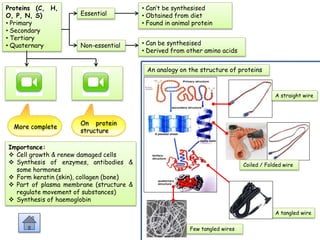
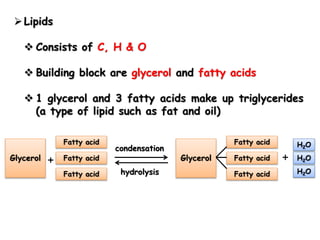
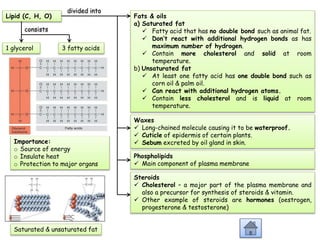
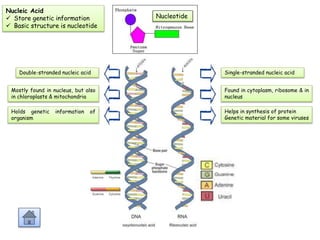
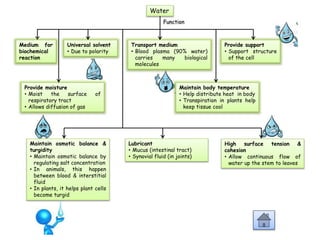
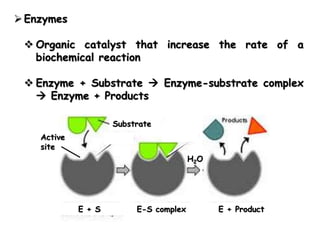
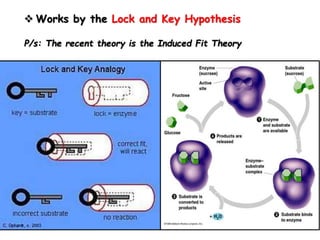
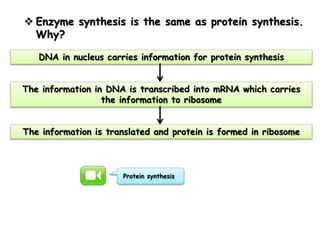
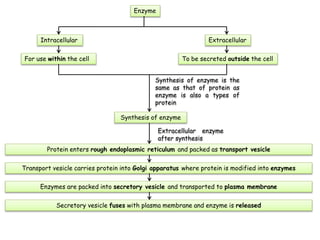
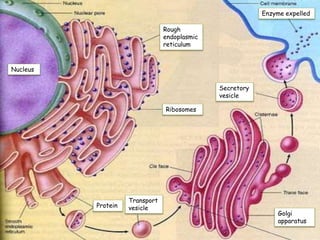
Cells contain various chemical compounds including ions, carbohydrates, proteins, lipids, nucleic acids, water, and enzymes. Carbohydrates include monosaccharides like glucose, disaccharides formed from two monosaccharides bonded together, and polysaccharides formed from many monosaccharides. Proteins are made of amino acids bonded together through peptide bonds. Lipids are made of glycerol and fatty acids and include fats, oils, and phospholipids. Nucleic acids like DNA and RNA contain nucleotides and store genetic information. Water acts as a solvent and transports molecules in cells. Enzymes are protein catalysts that speed up biochemical reactions.