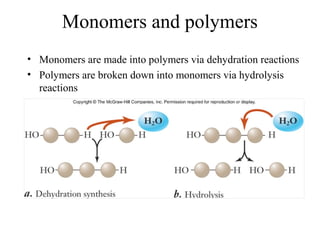
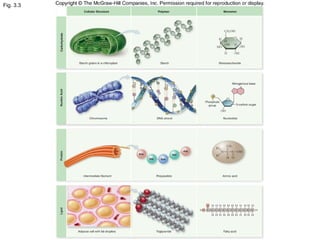
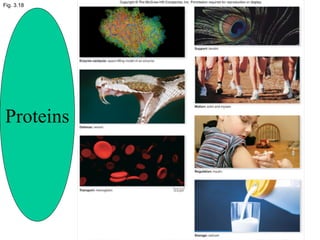
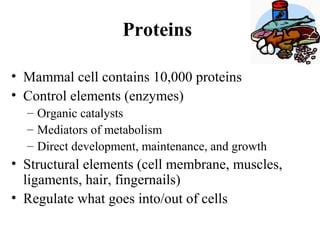
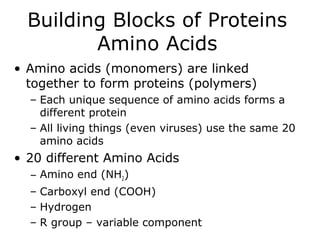
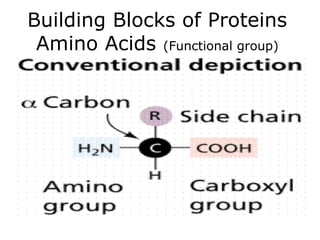
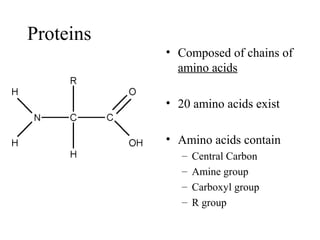
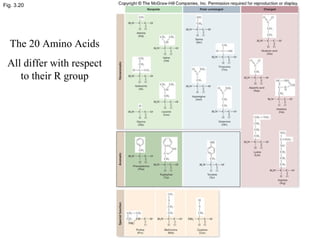
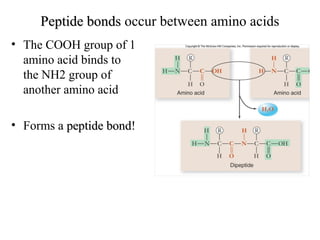
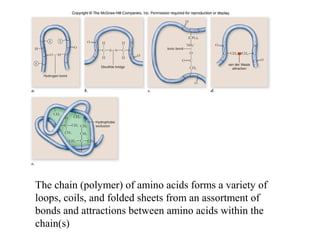
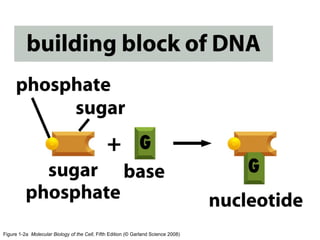
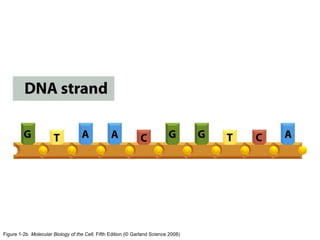
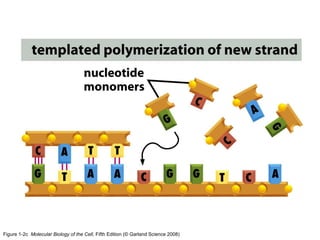
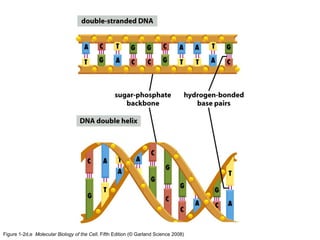
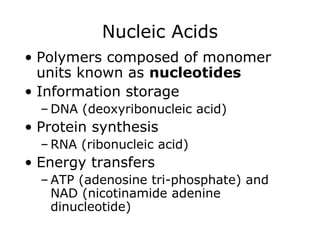
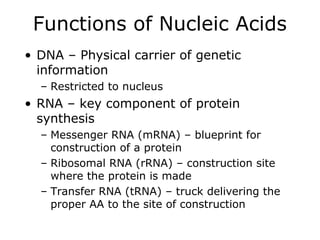
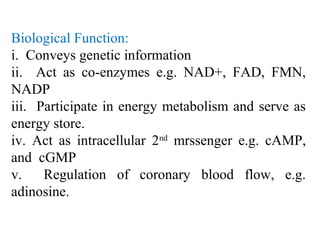
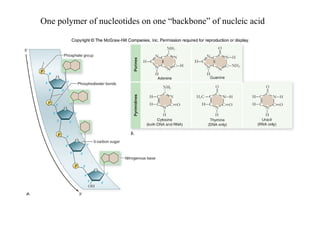
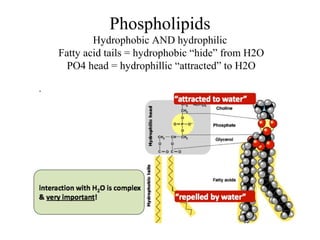
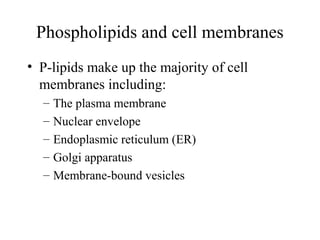
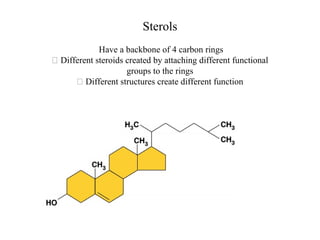
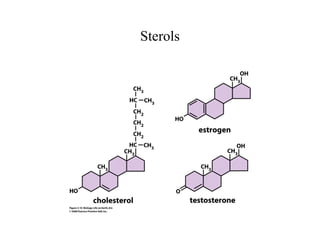
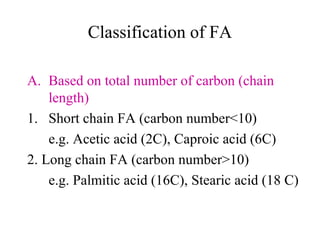
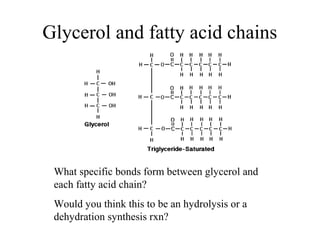
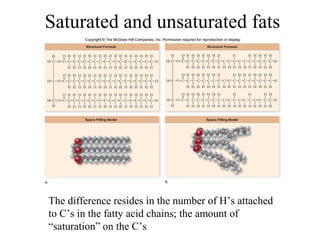
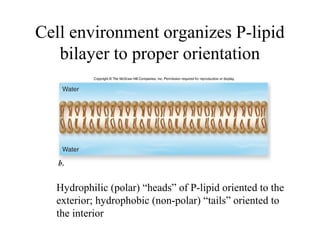
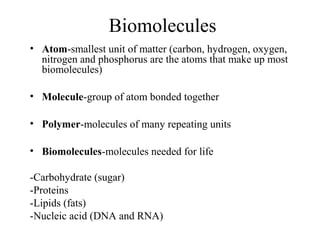
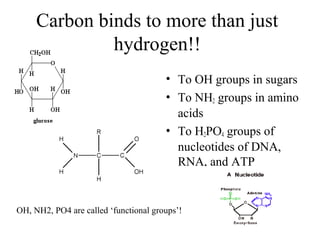
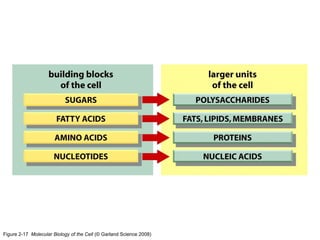
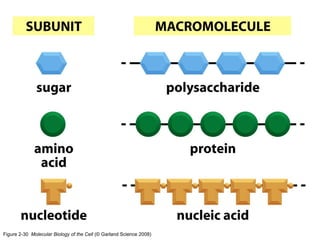
The document discusses the four main types of biomolecules - carbohydrates, lipids, proteins, and nucleic acids. It provides details on the monomers, polymers, and functions of each type. Carbohydrates include sugars such as glucose and polymers like starch. Lipids are made of fatty acids and include fats, waxes, and phospholipids. Proteins are made of amino acid polymers that take on various structures and functions. Nucleic acids like DNA and RNA are composed of nucleotides and carry genetic information.


![Carbohydrates
• Carbohydrate contain aldehyde (CHO)
and ketone (-c o) functional group.꞊
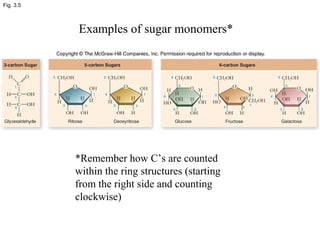
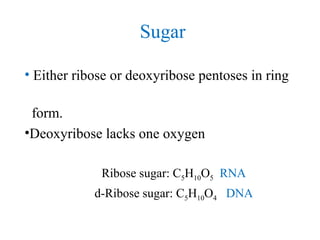
• Monosaccharides:[cn(H2O)n]
•Five carbone: Ribose
•Six carbone: Glucose and fructose](https://image.slidesharecdn.com/2-180810154111/85/2-biomolecule-10-320.jpg)
![Carbohydrates
• Disaccharides [cn(H2O)n]
–Sucrose (glucose+glucose)
–Lactose (glucose+galactose)
–Maltose (glucose+fructose)
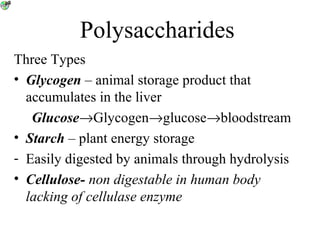
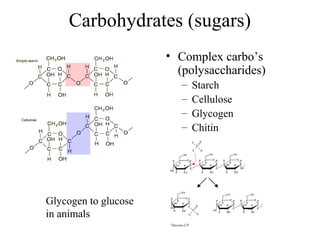
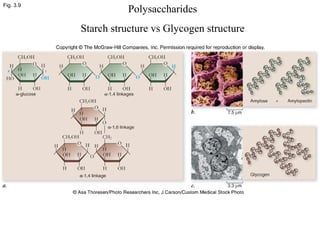
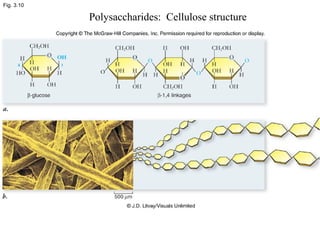
• Polysaccharides [c6(H10O)5]n
–Starch
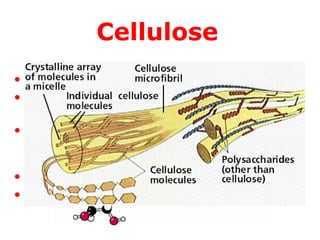
–Cellulose
–Glycogen
• Oligosaccharides:](https://image.slidesharecdn.com/2-180810154111/85/2-biomolecule-11-320.jpg)