This document provides an overview of the syllabus for an Anatomy and Physiology I course. It includes:
1. Contact information for the instructor and an outline of topics to be covered in Unit I, including cell chemistry, physiological processes, and homeostasis.
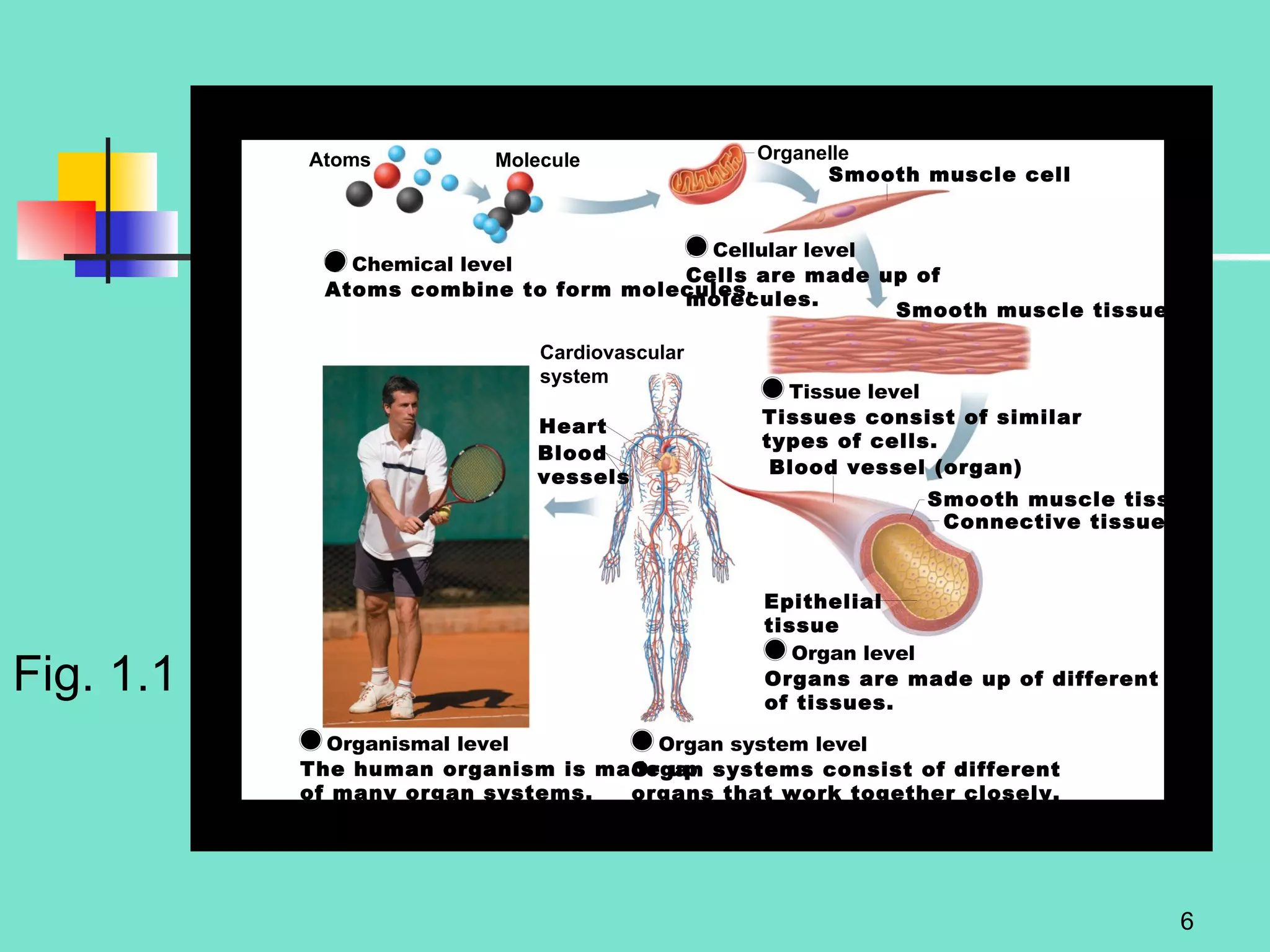
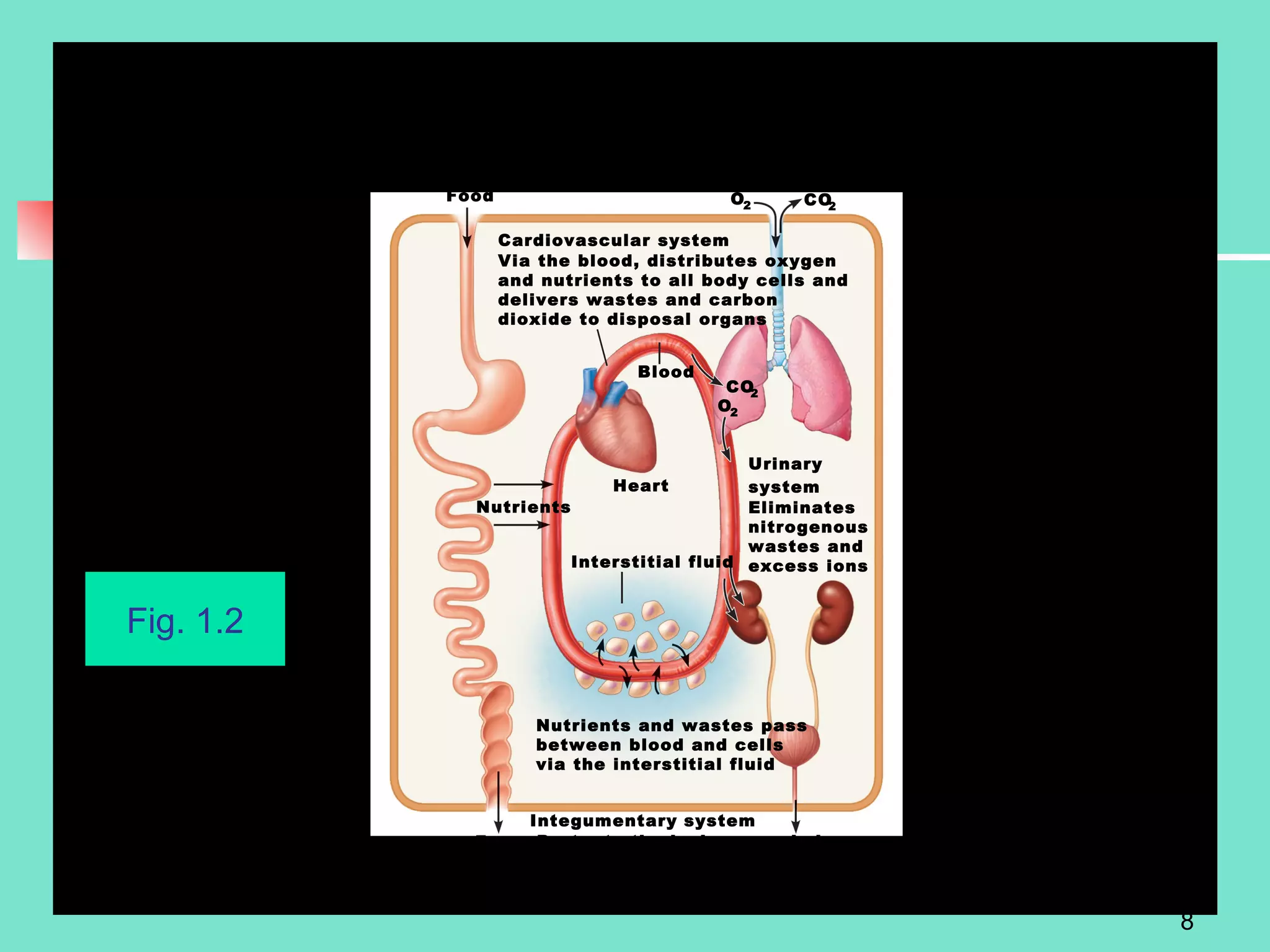
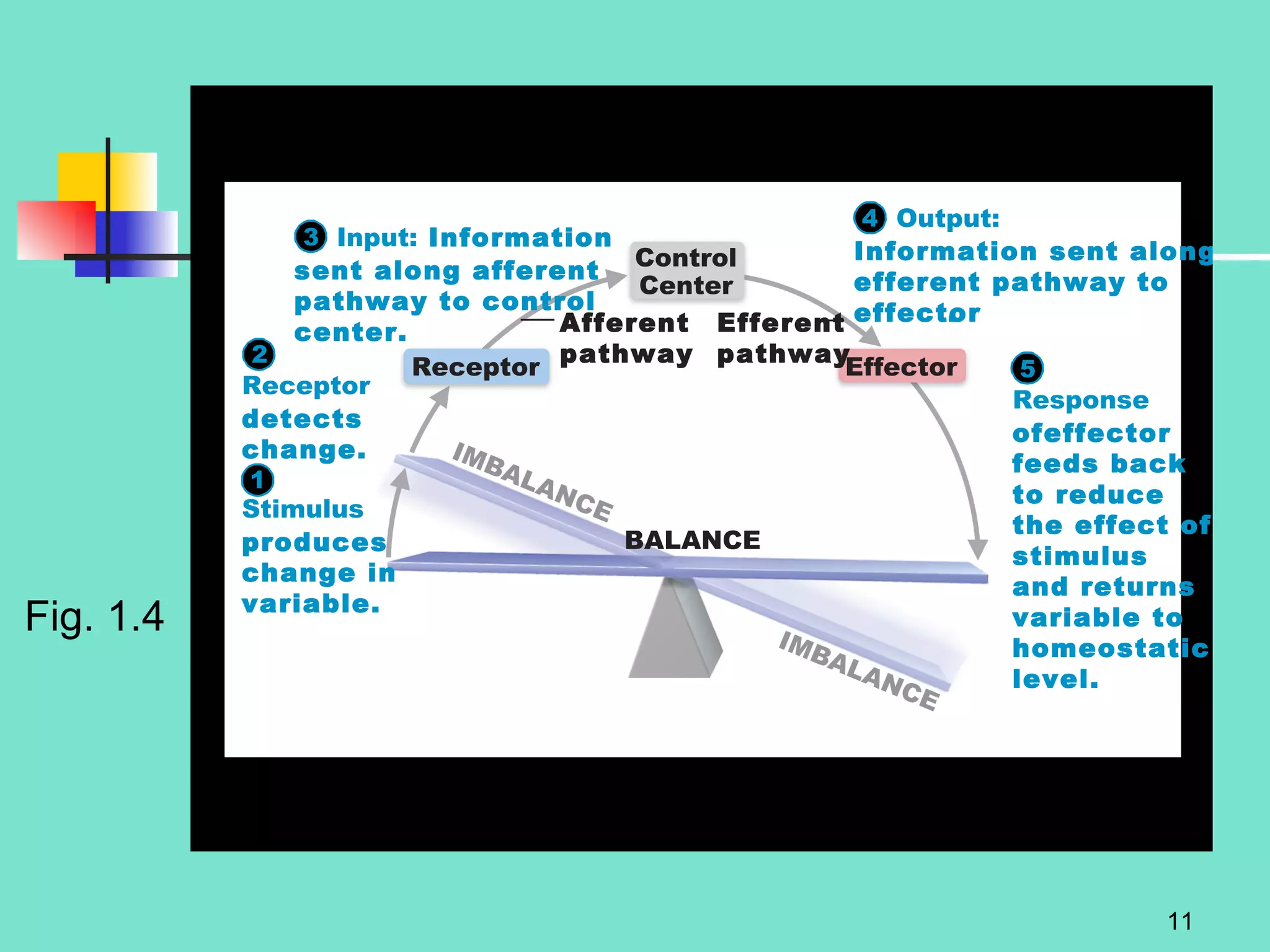
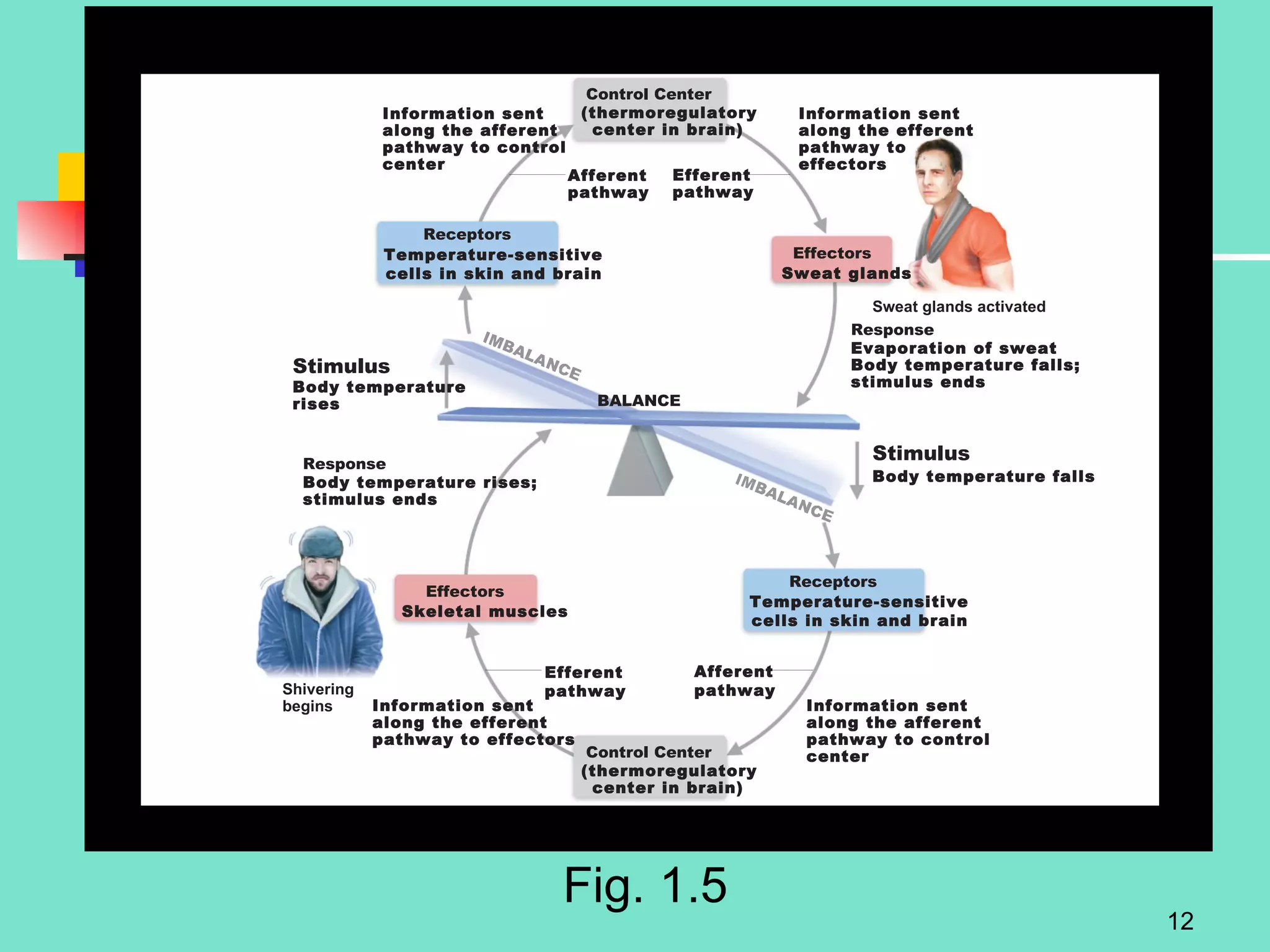
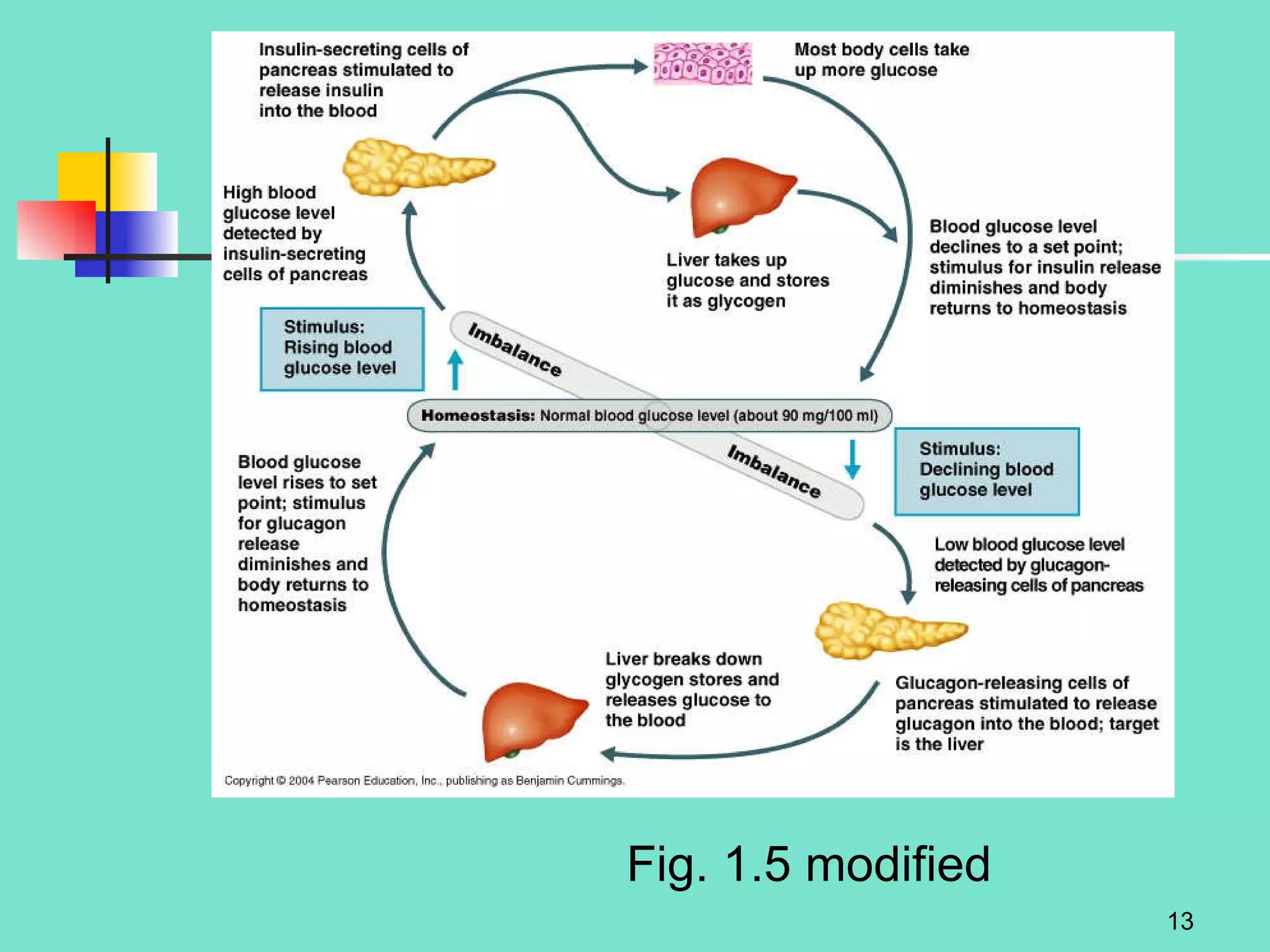
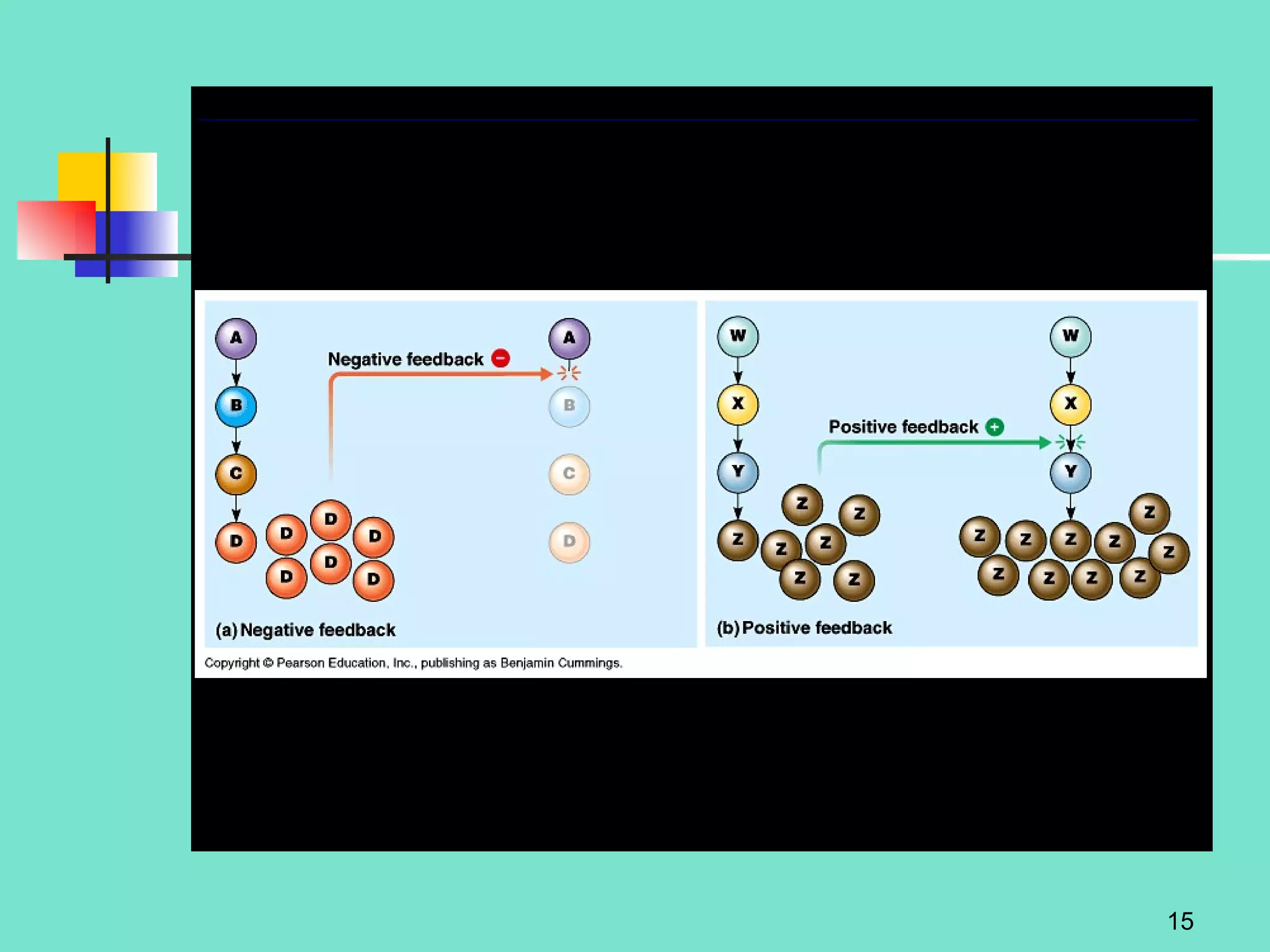
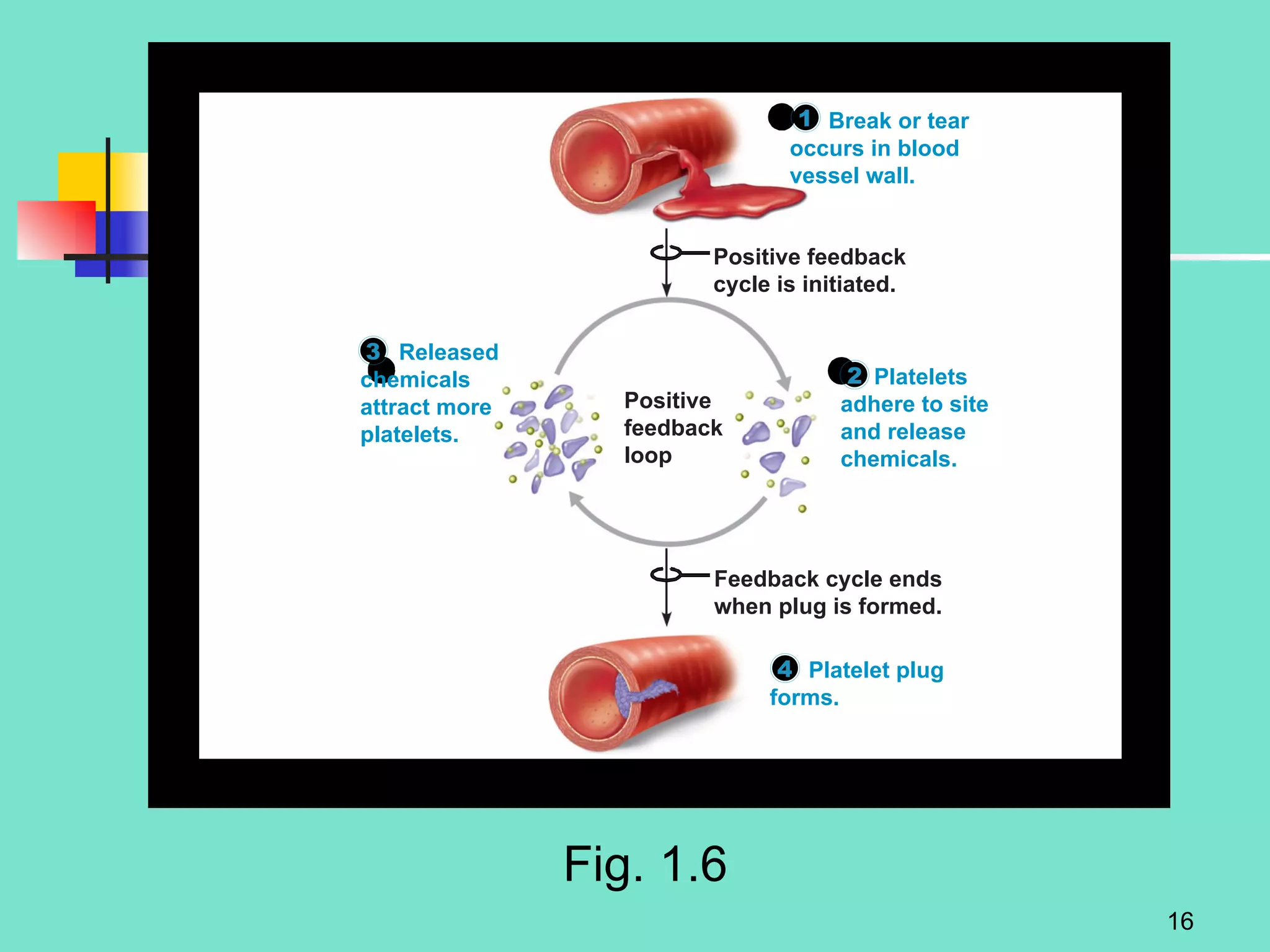
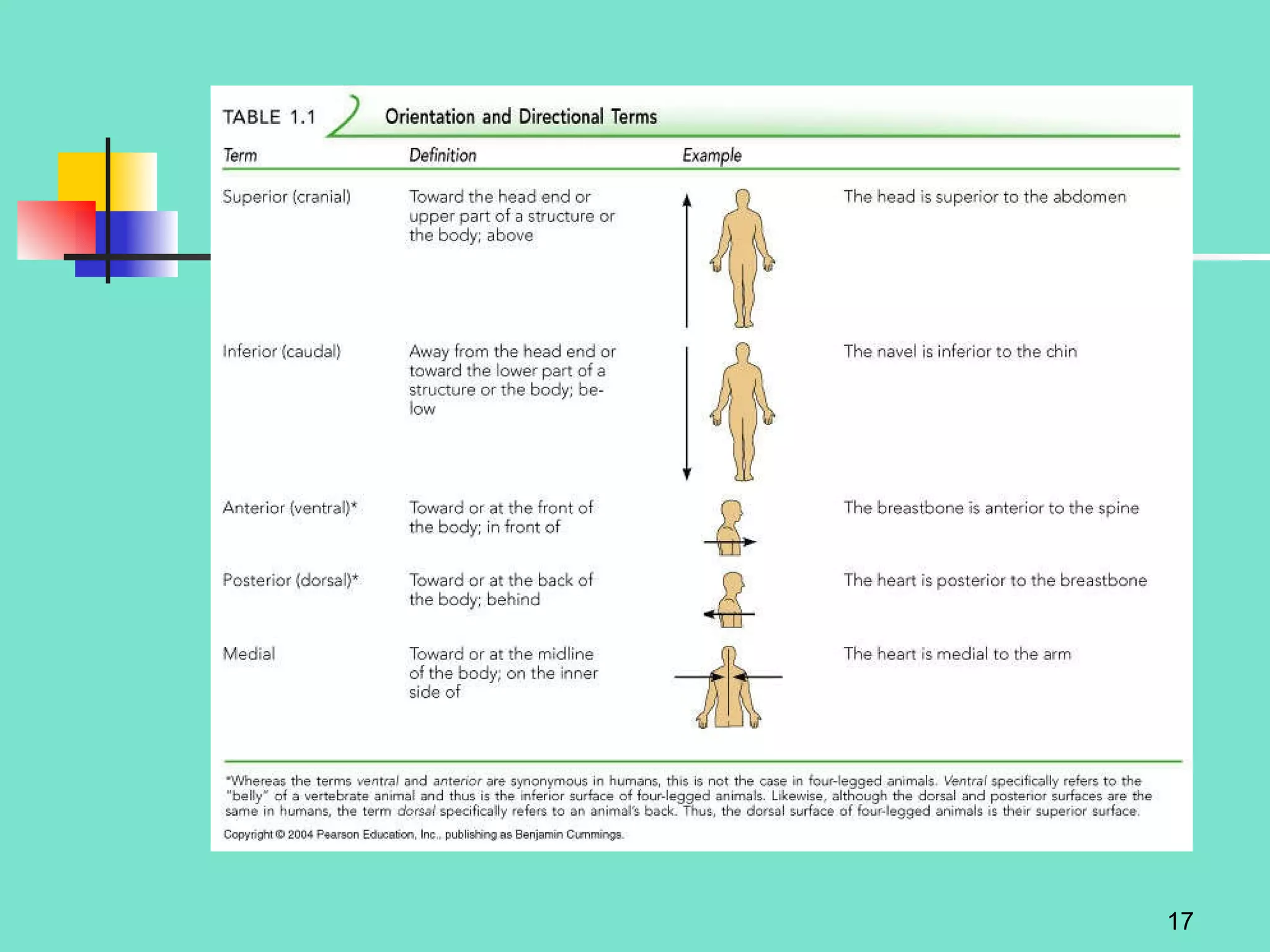
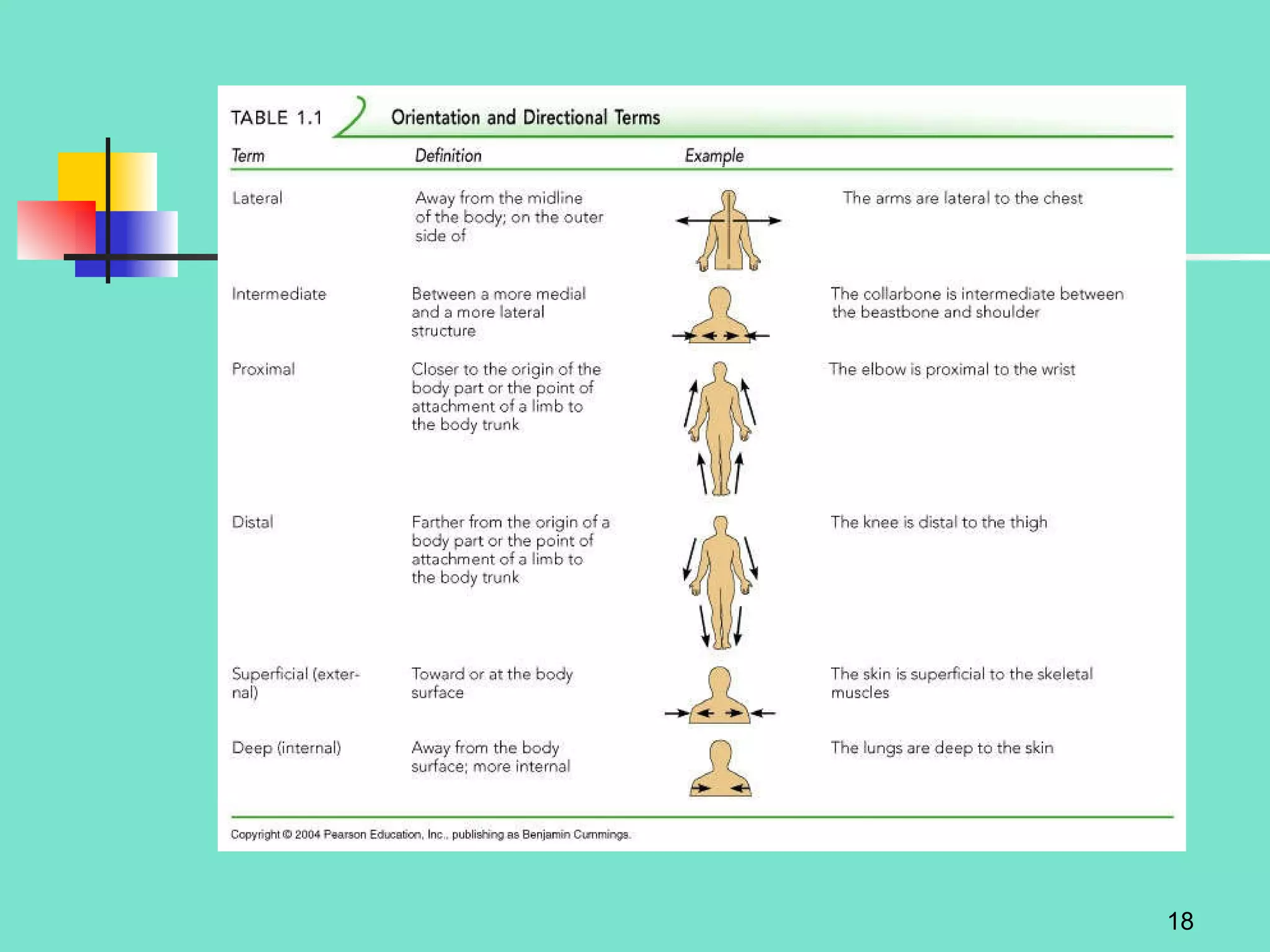
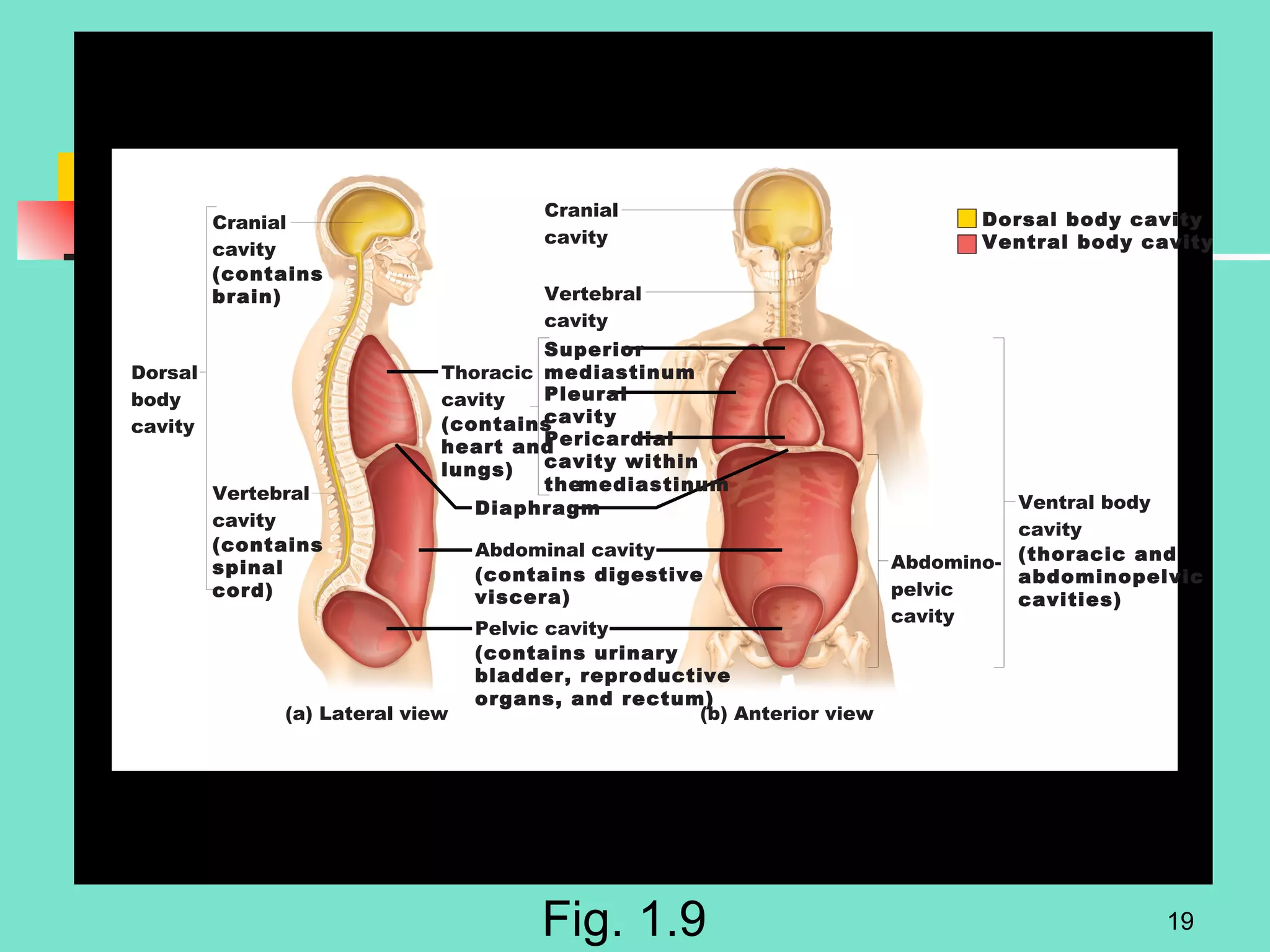
2. Descriptions of the basic components of life, including cells, tissues, organs and systems. It also discusses metabolism, homeostasis, and stress responses.
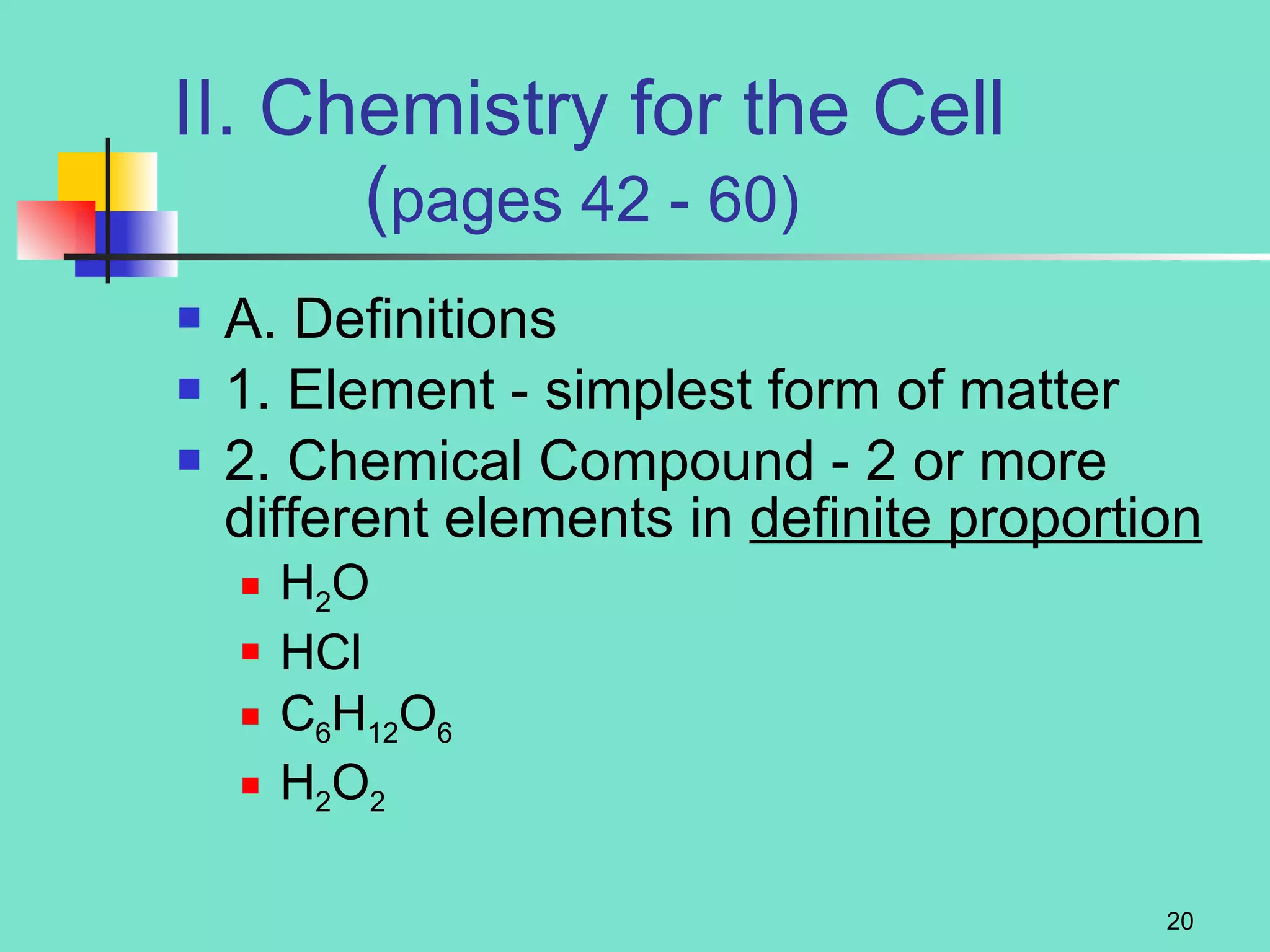
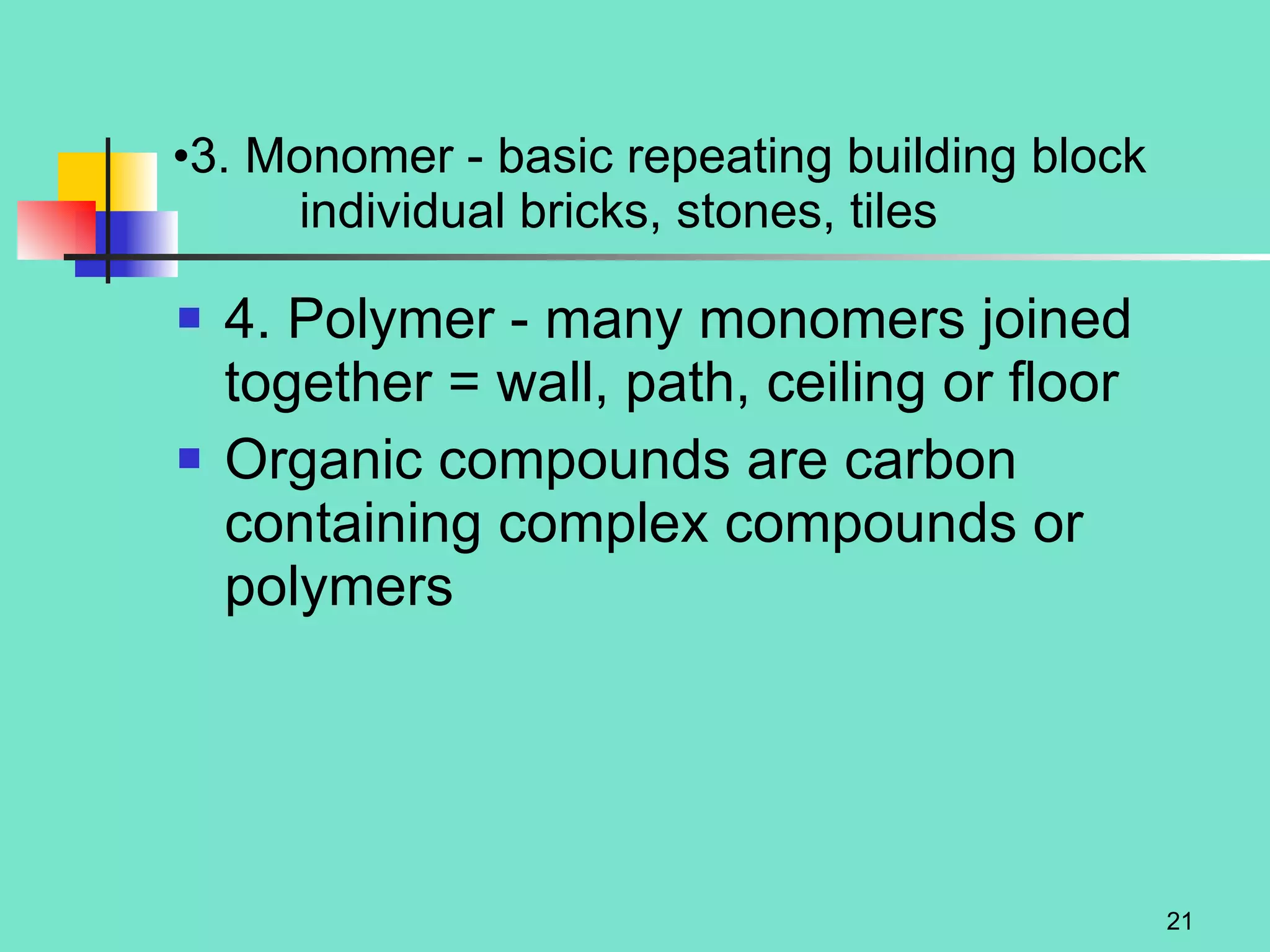
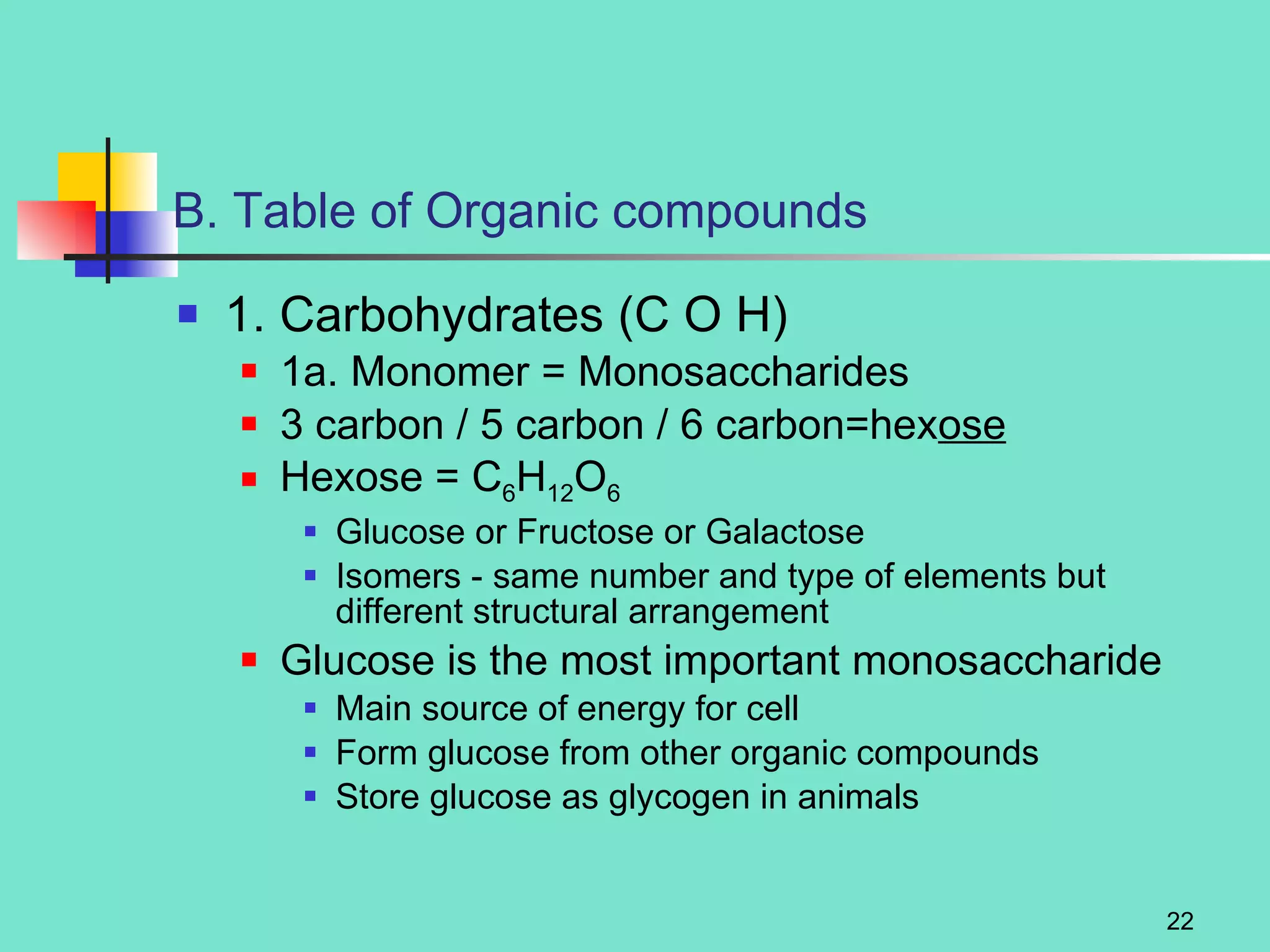
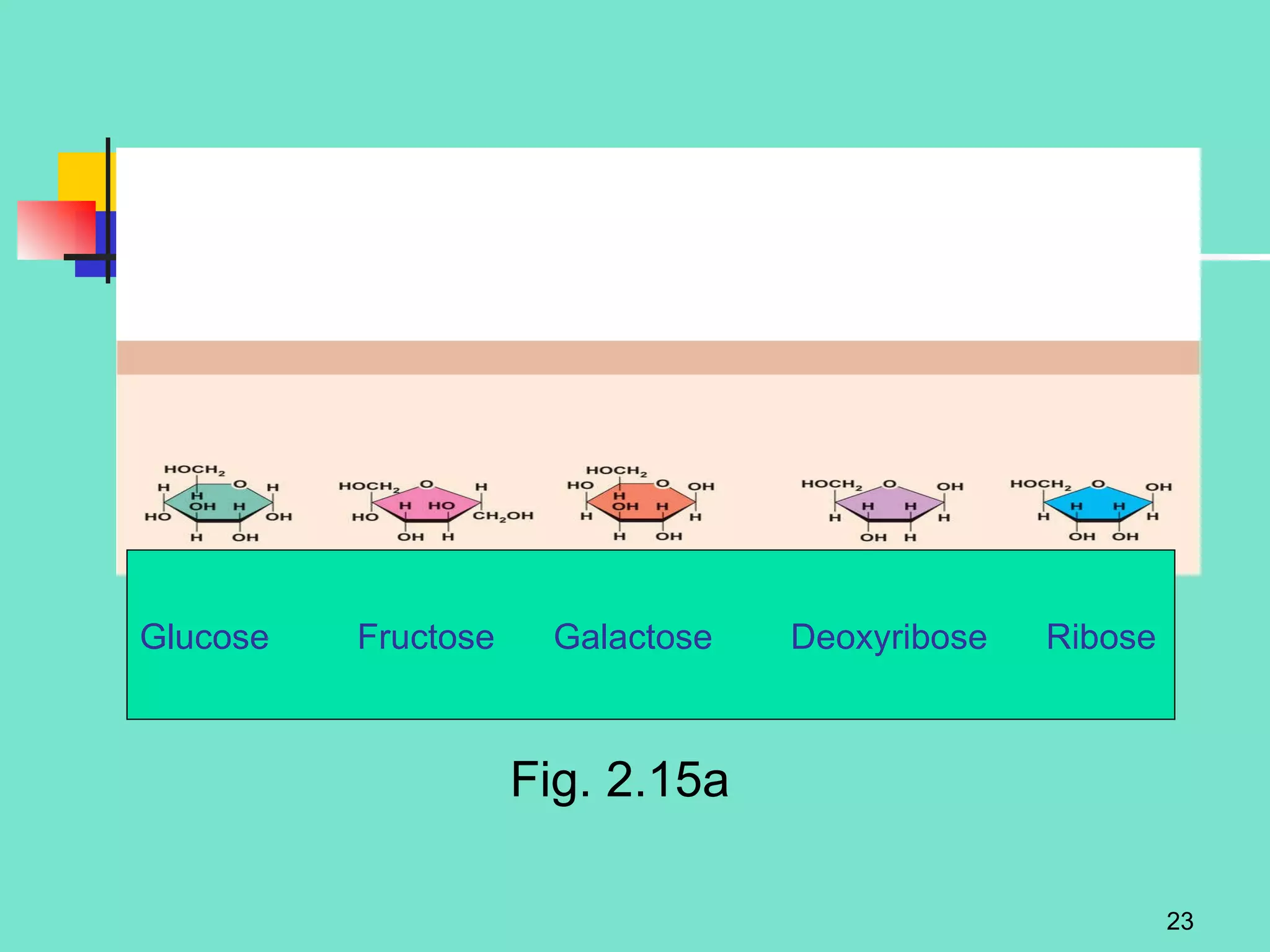
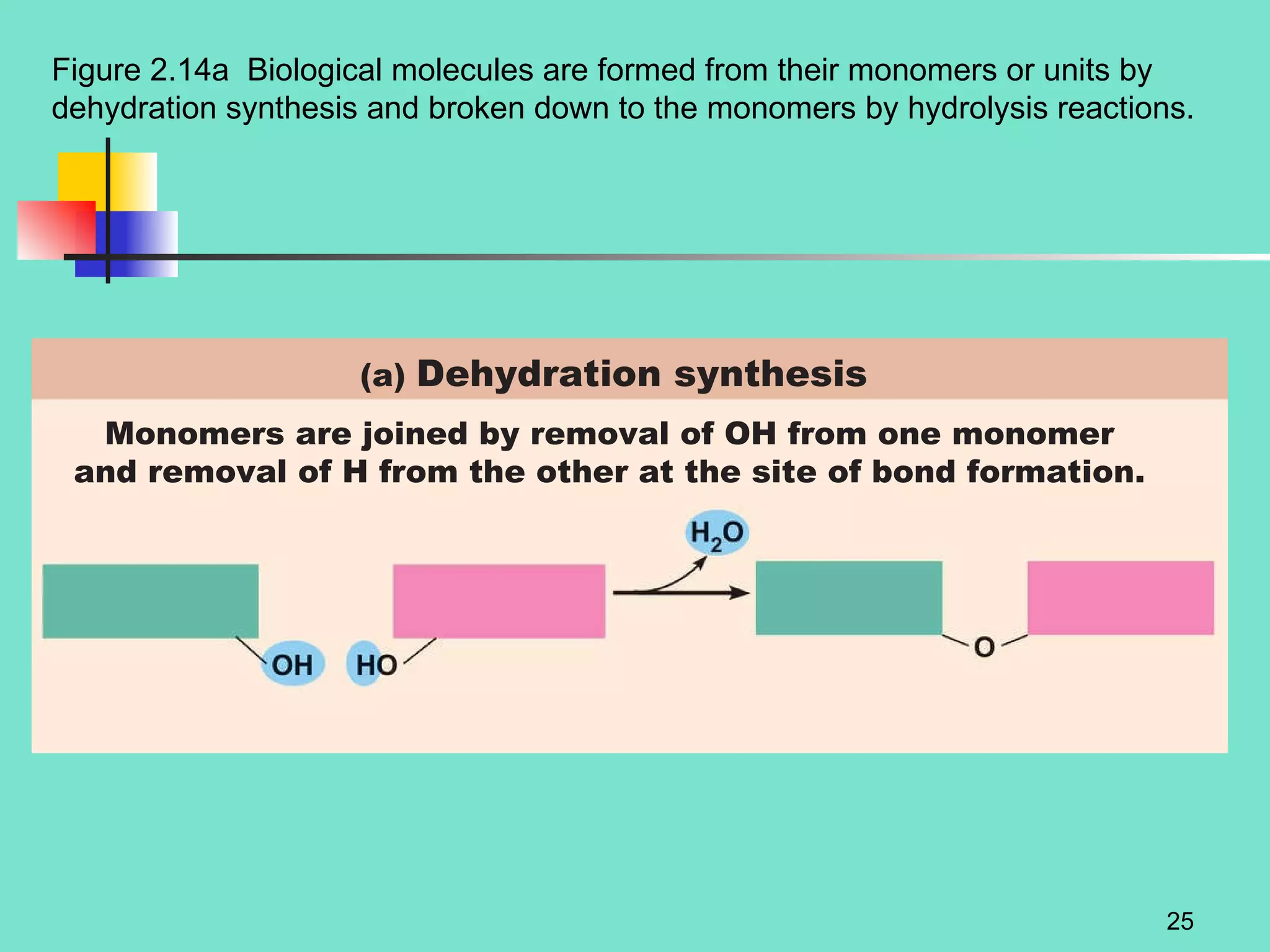
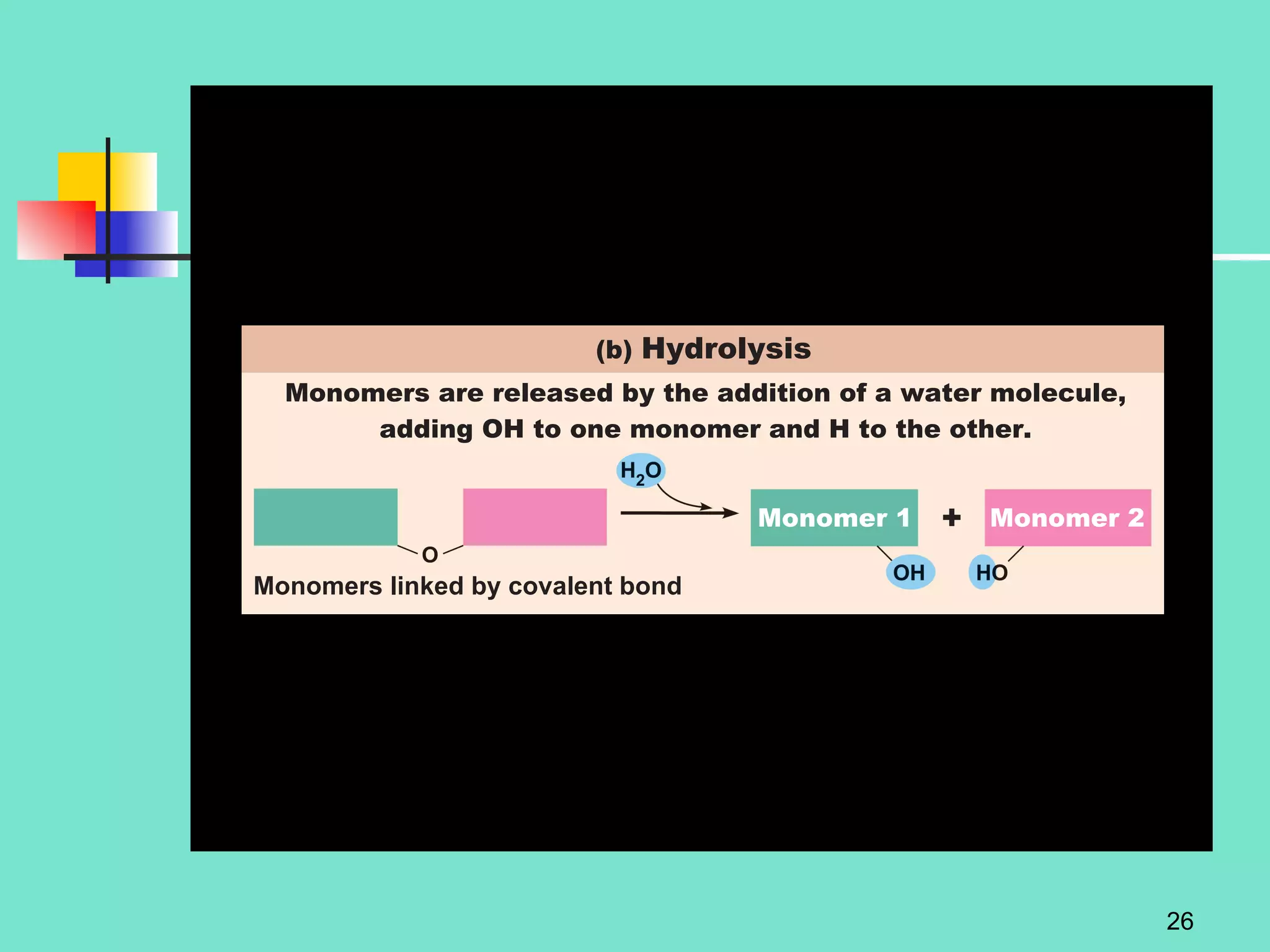
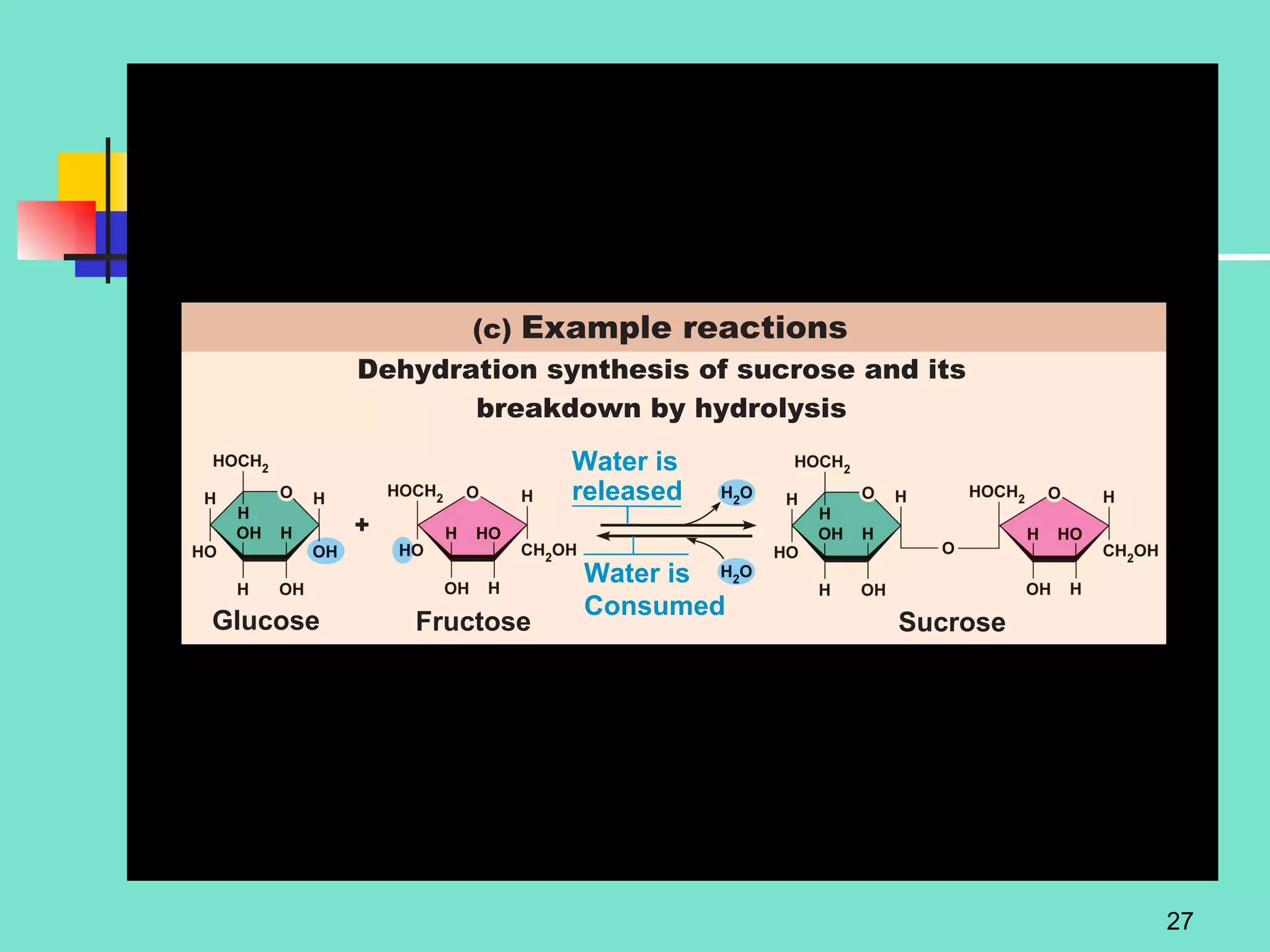
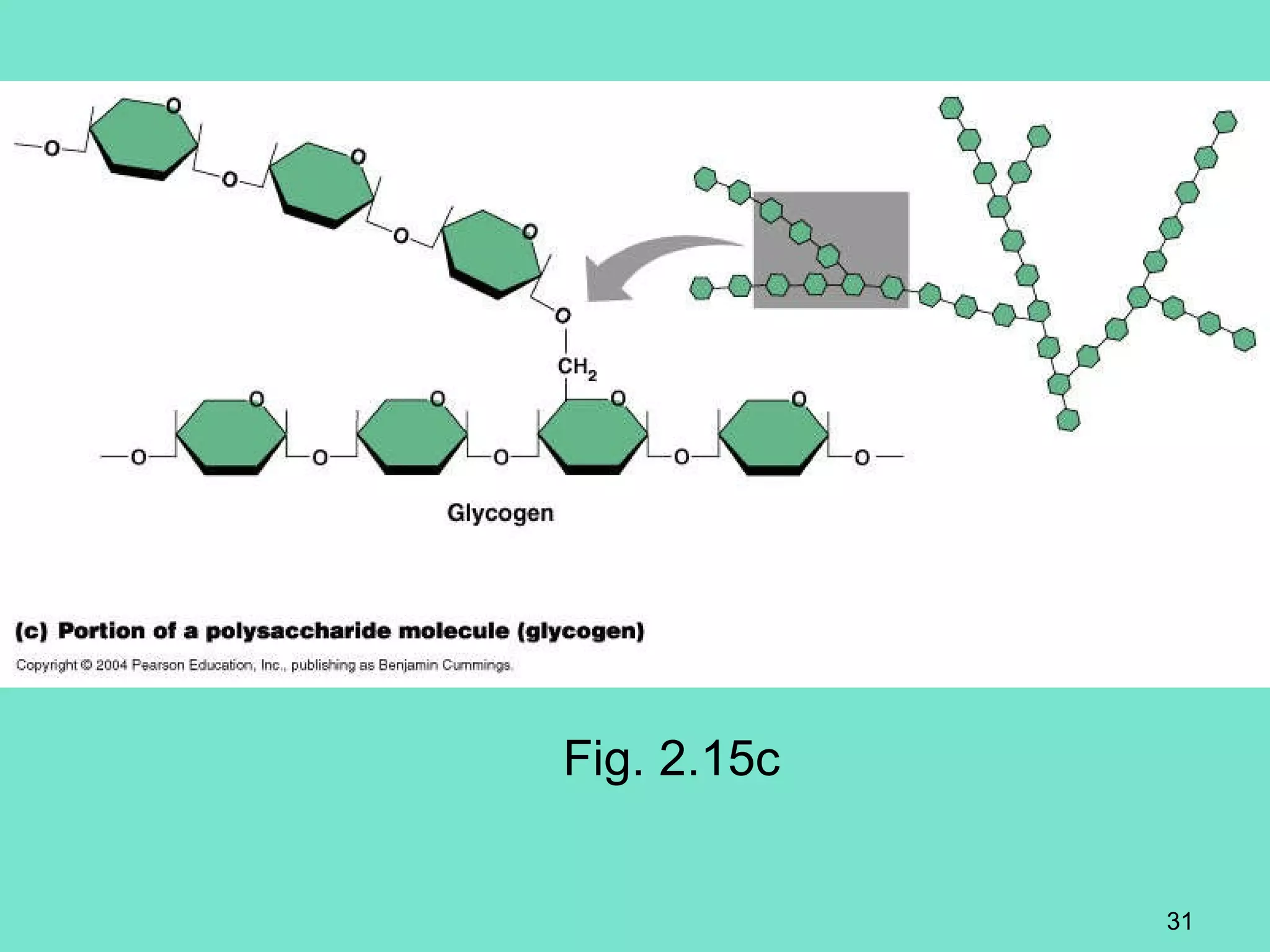
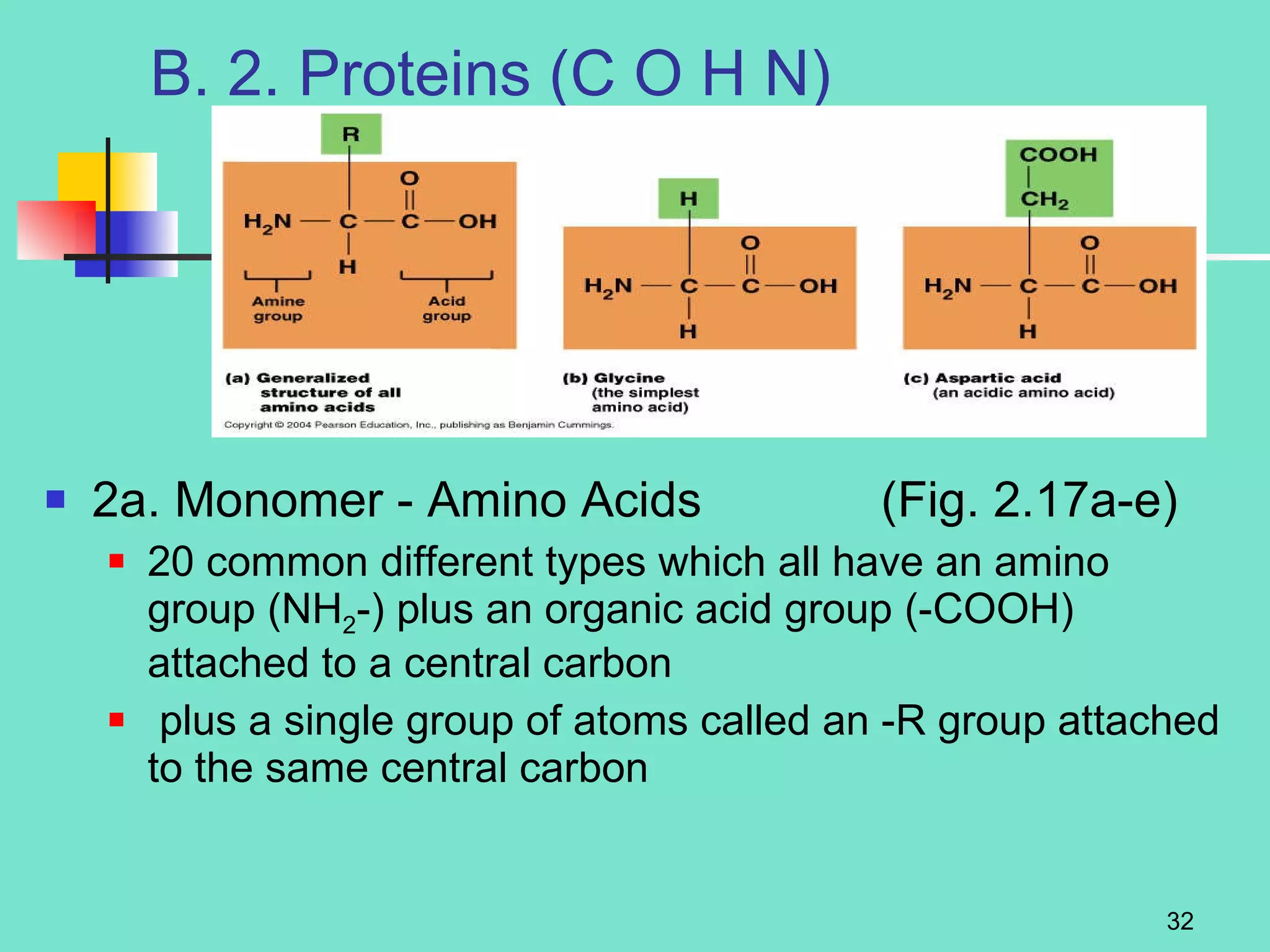
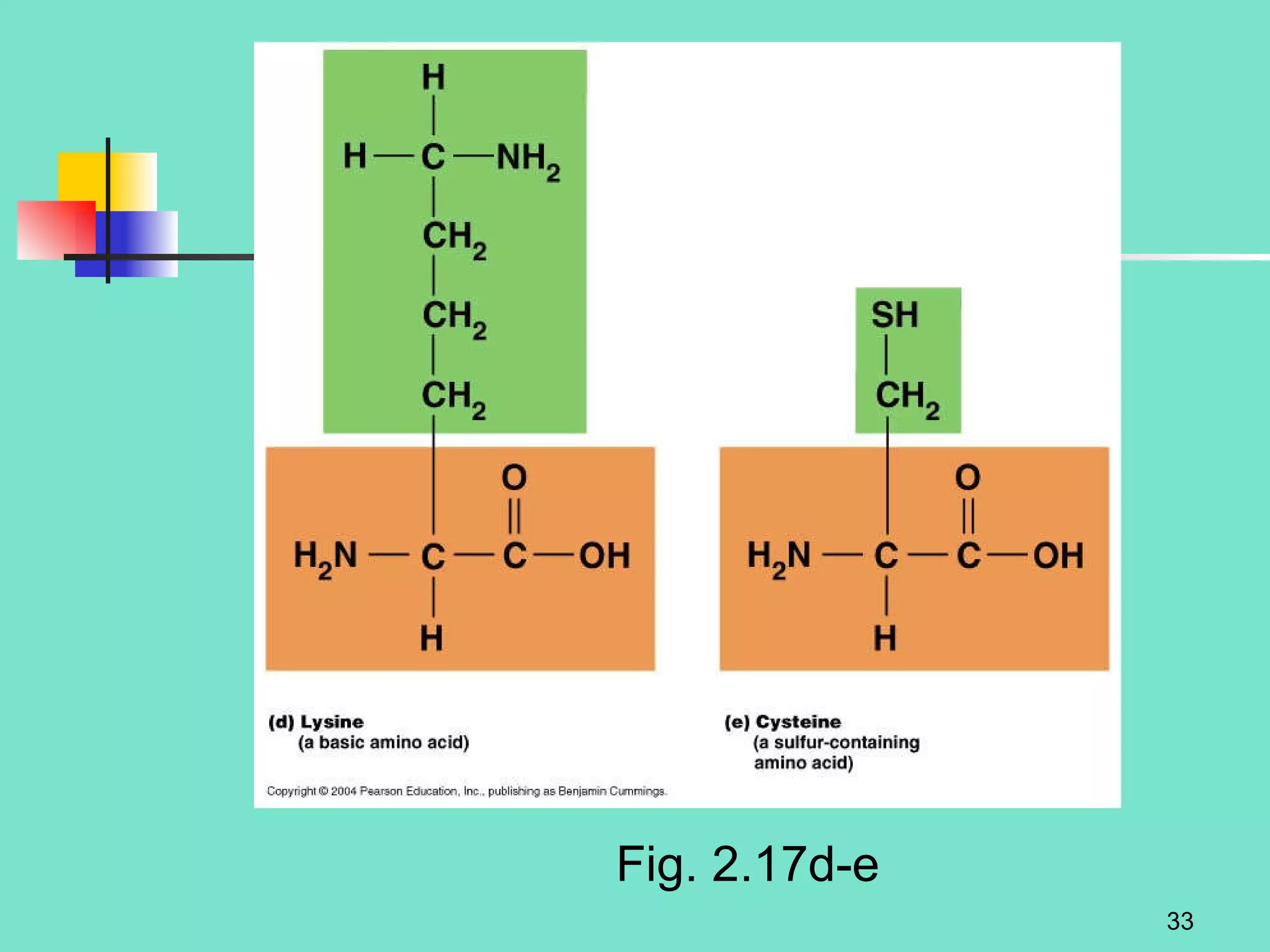
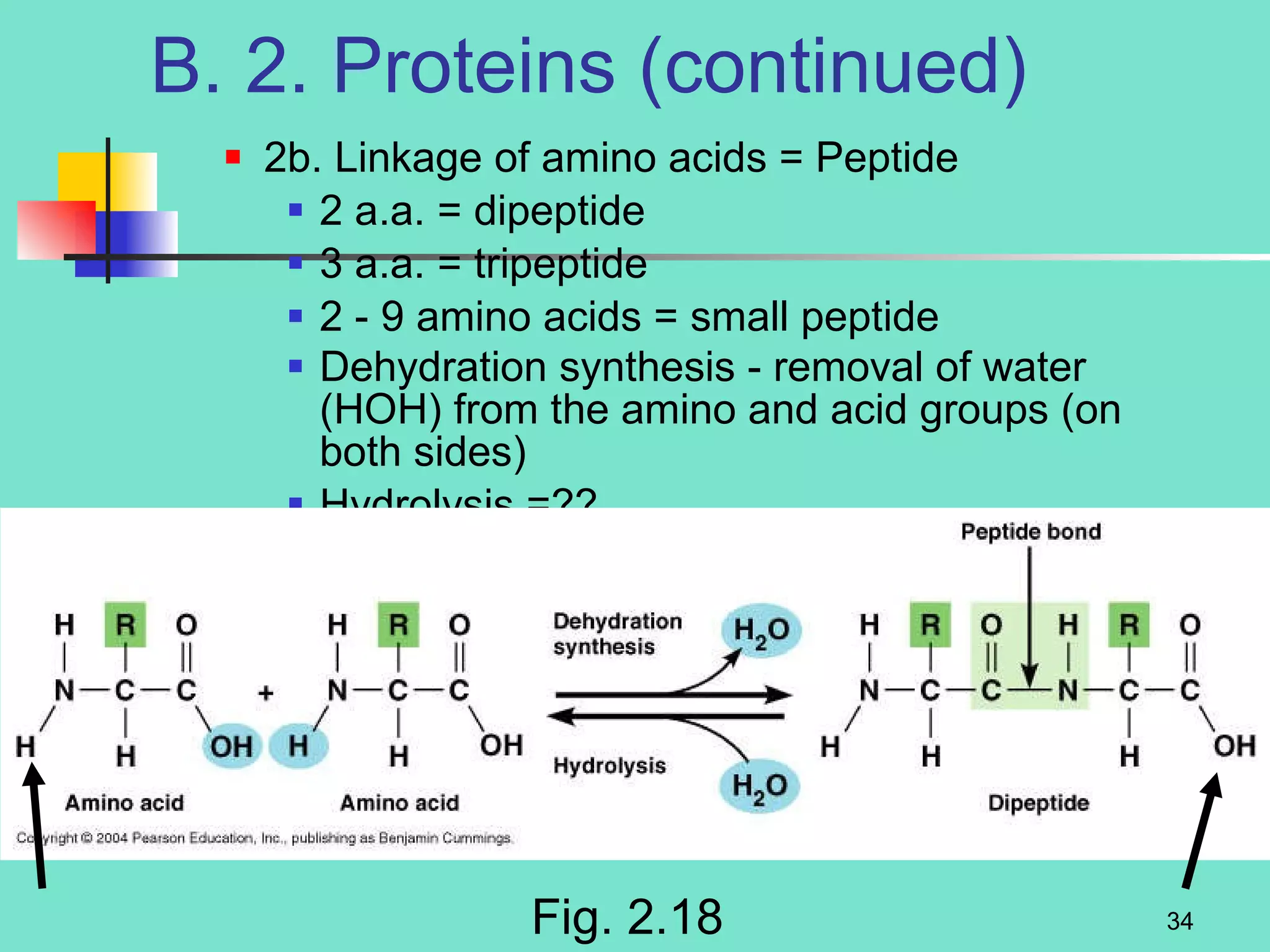
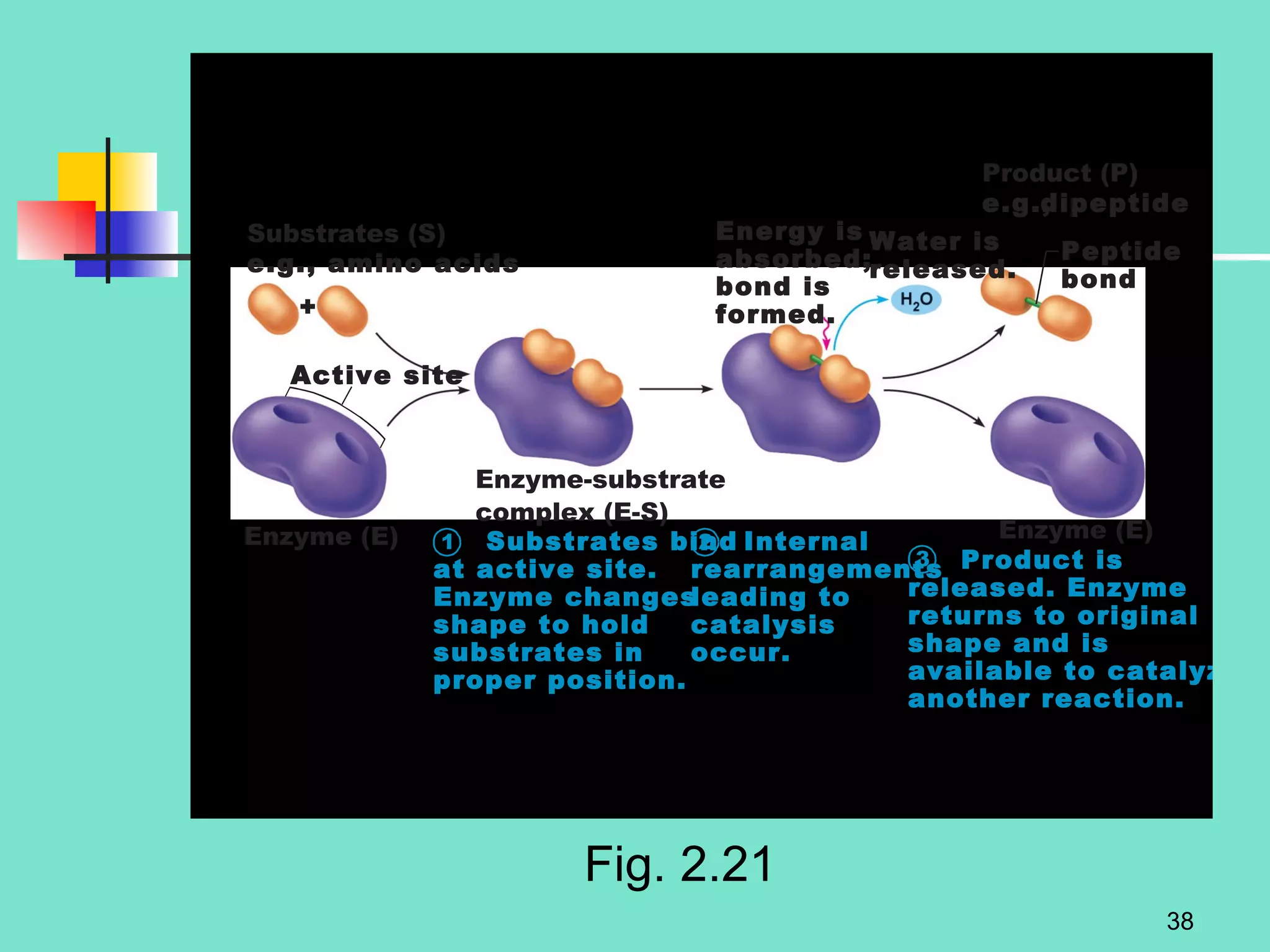
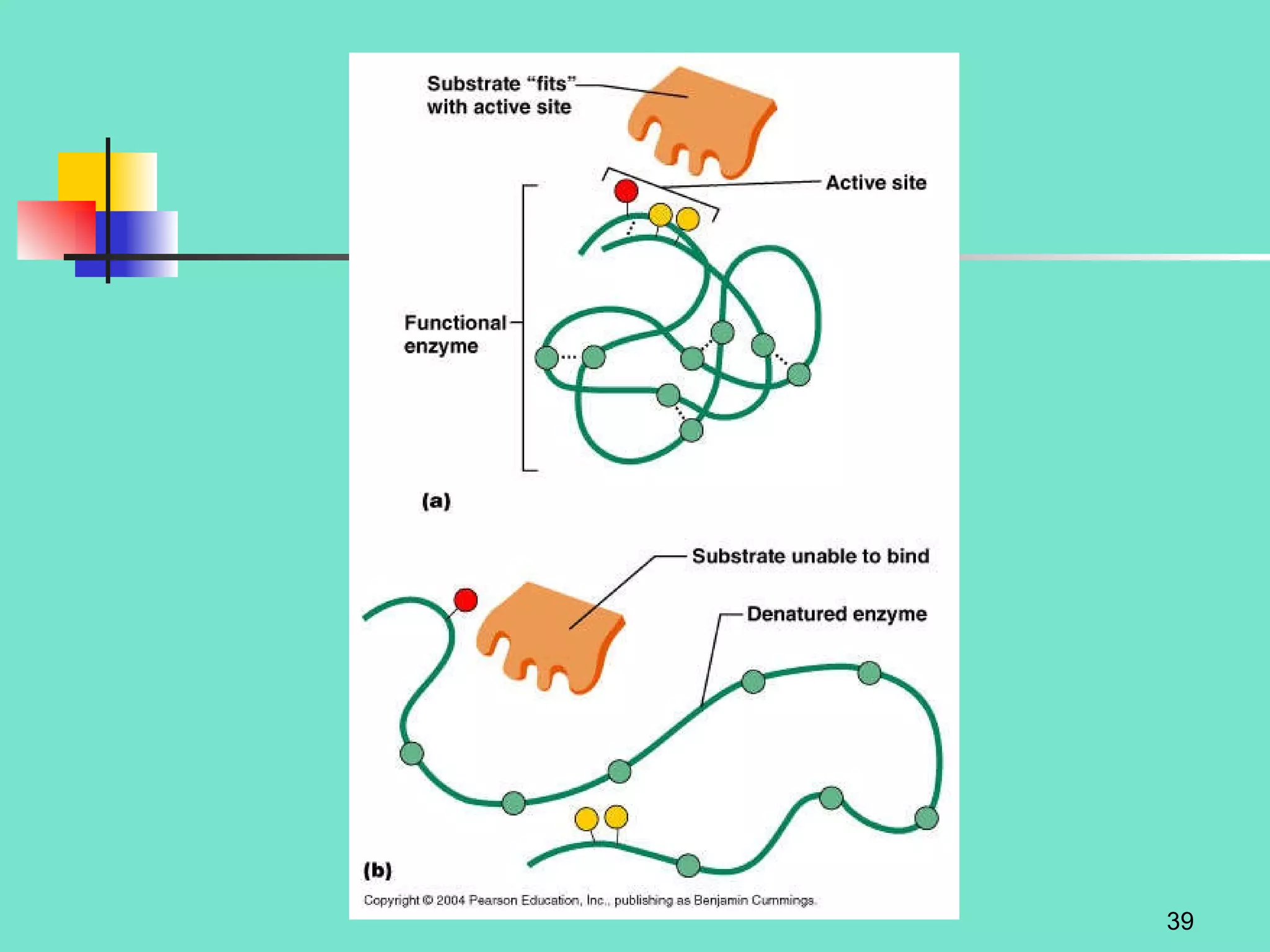
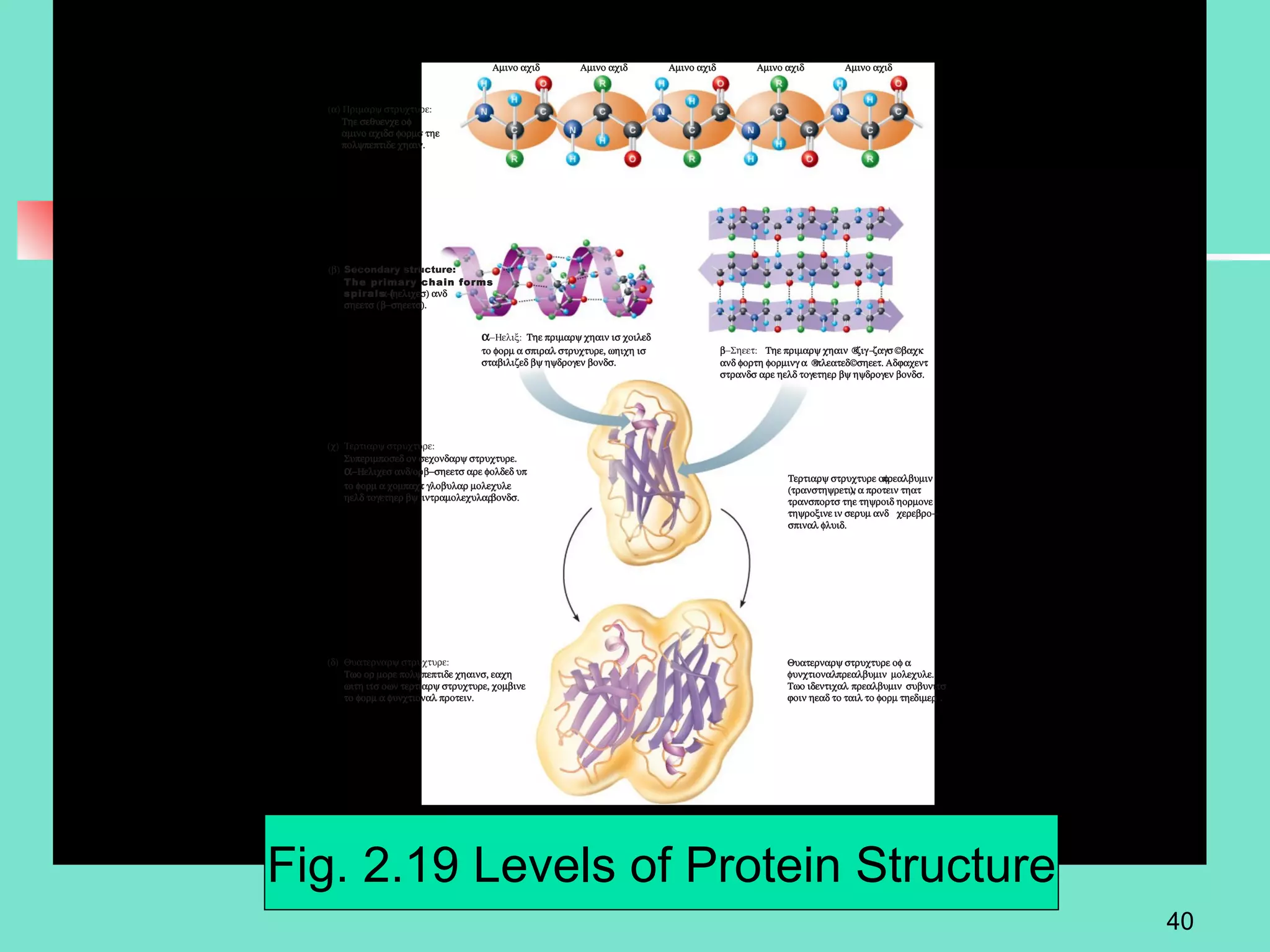
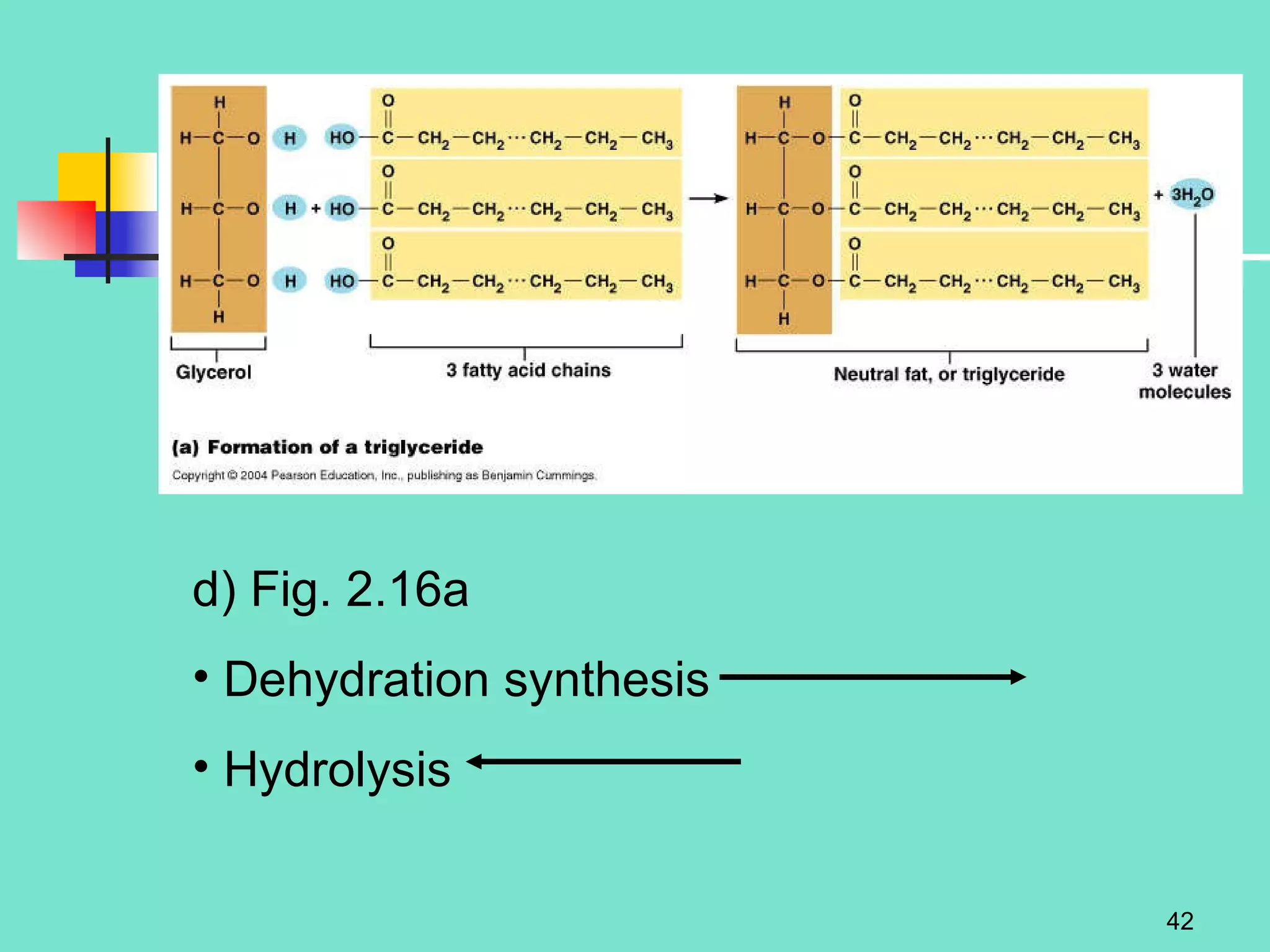
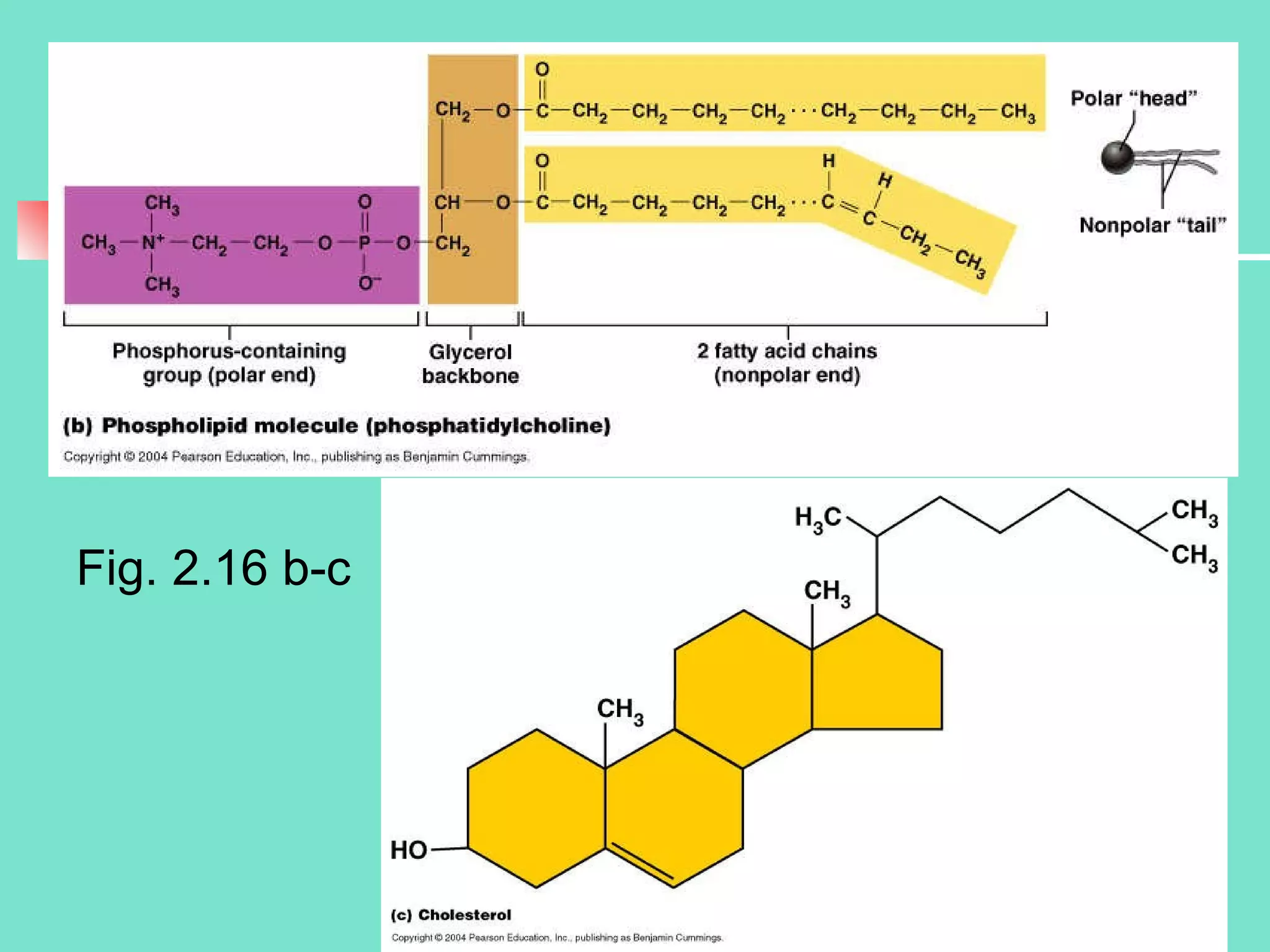
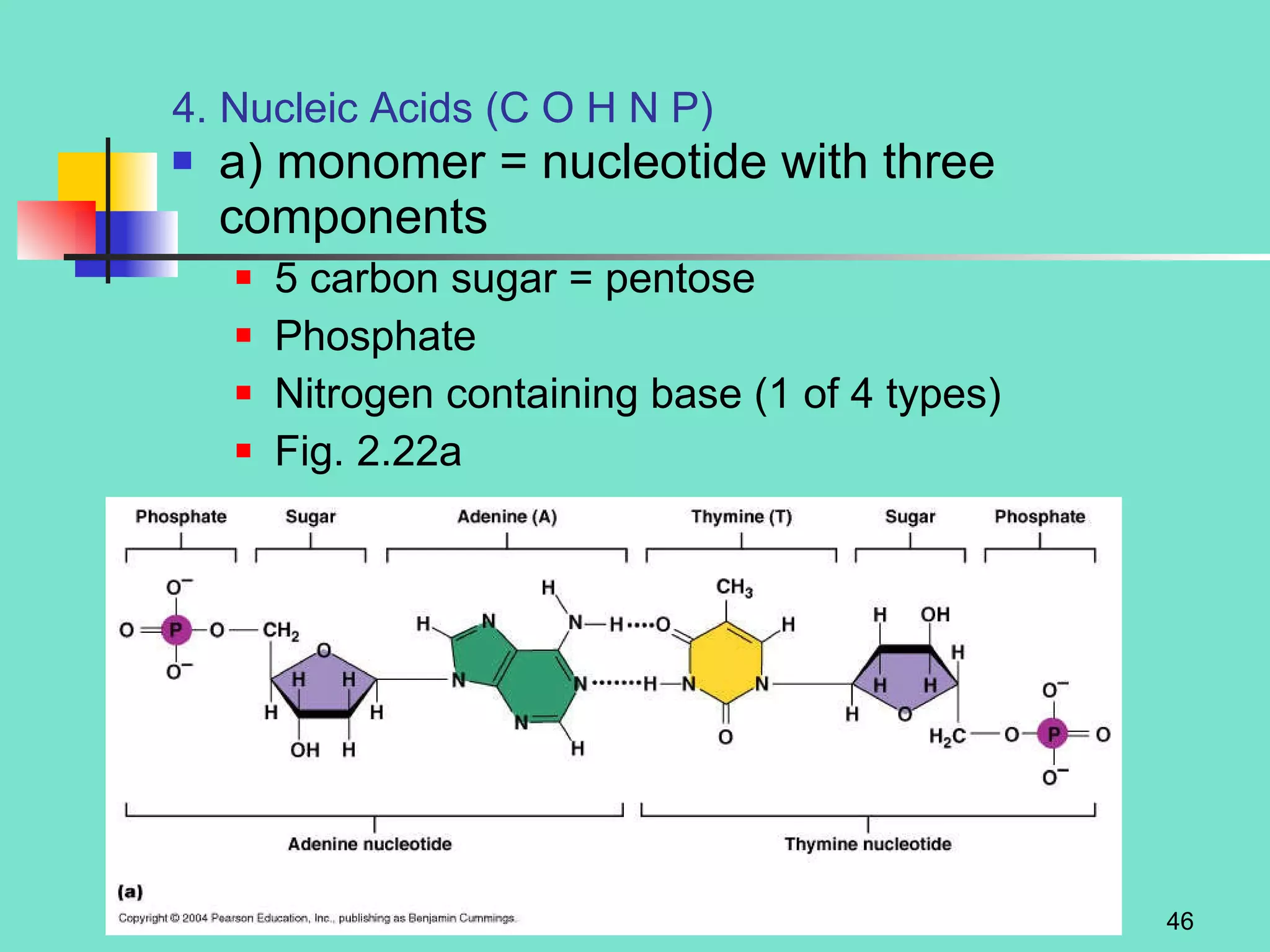
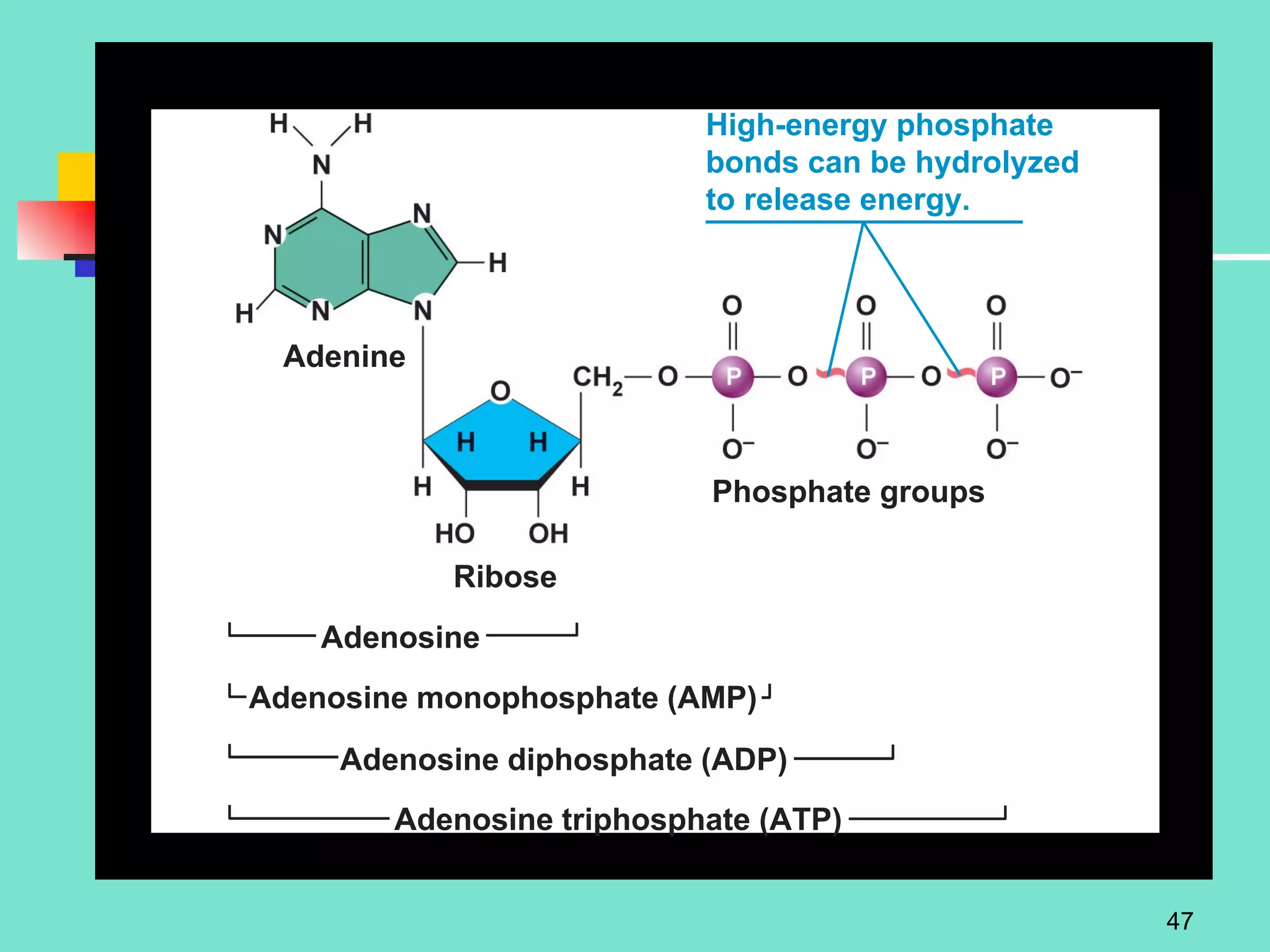
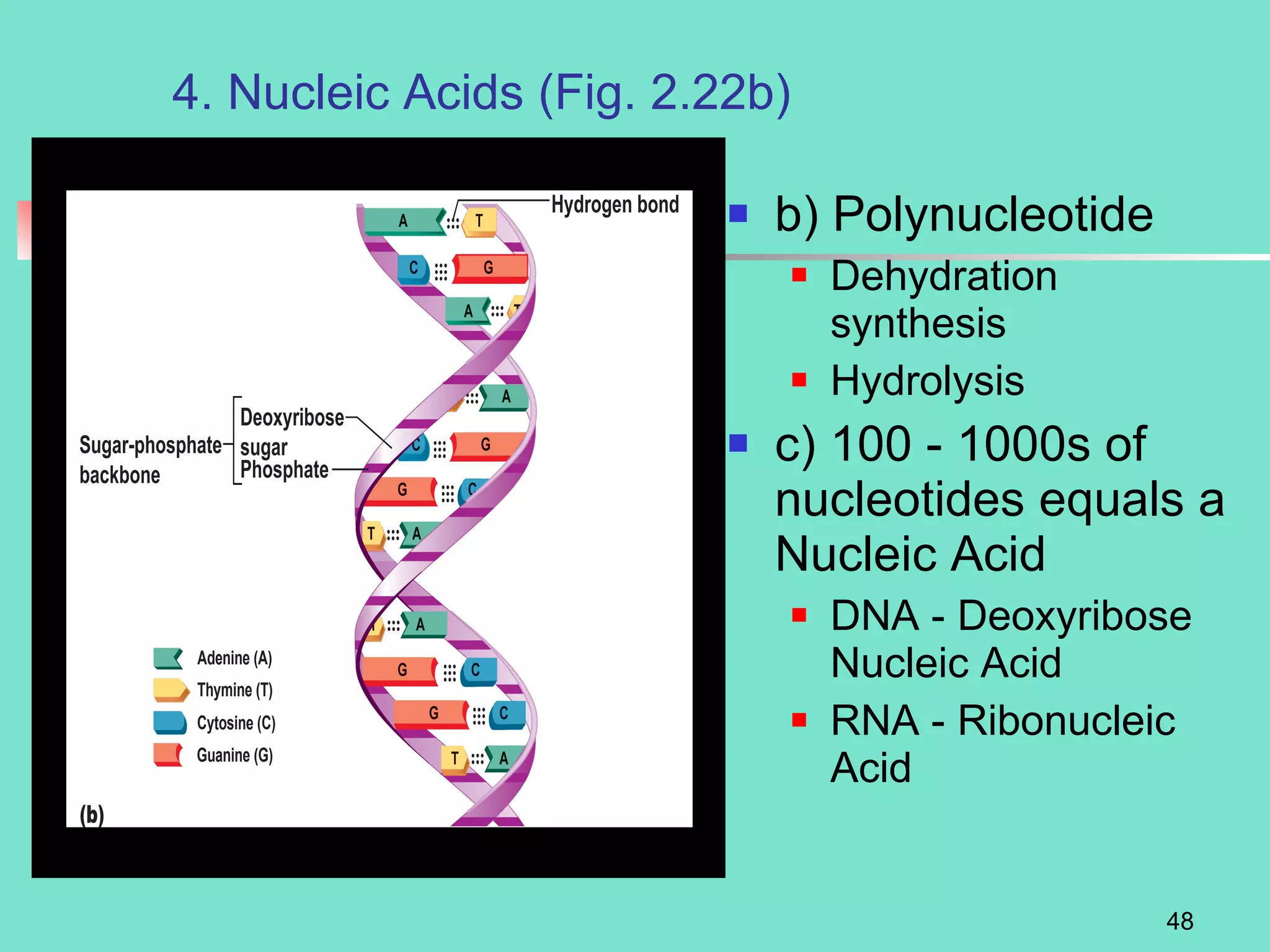
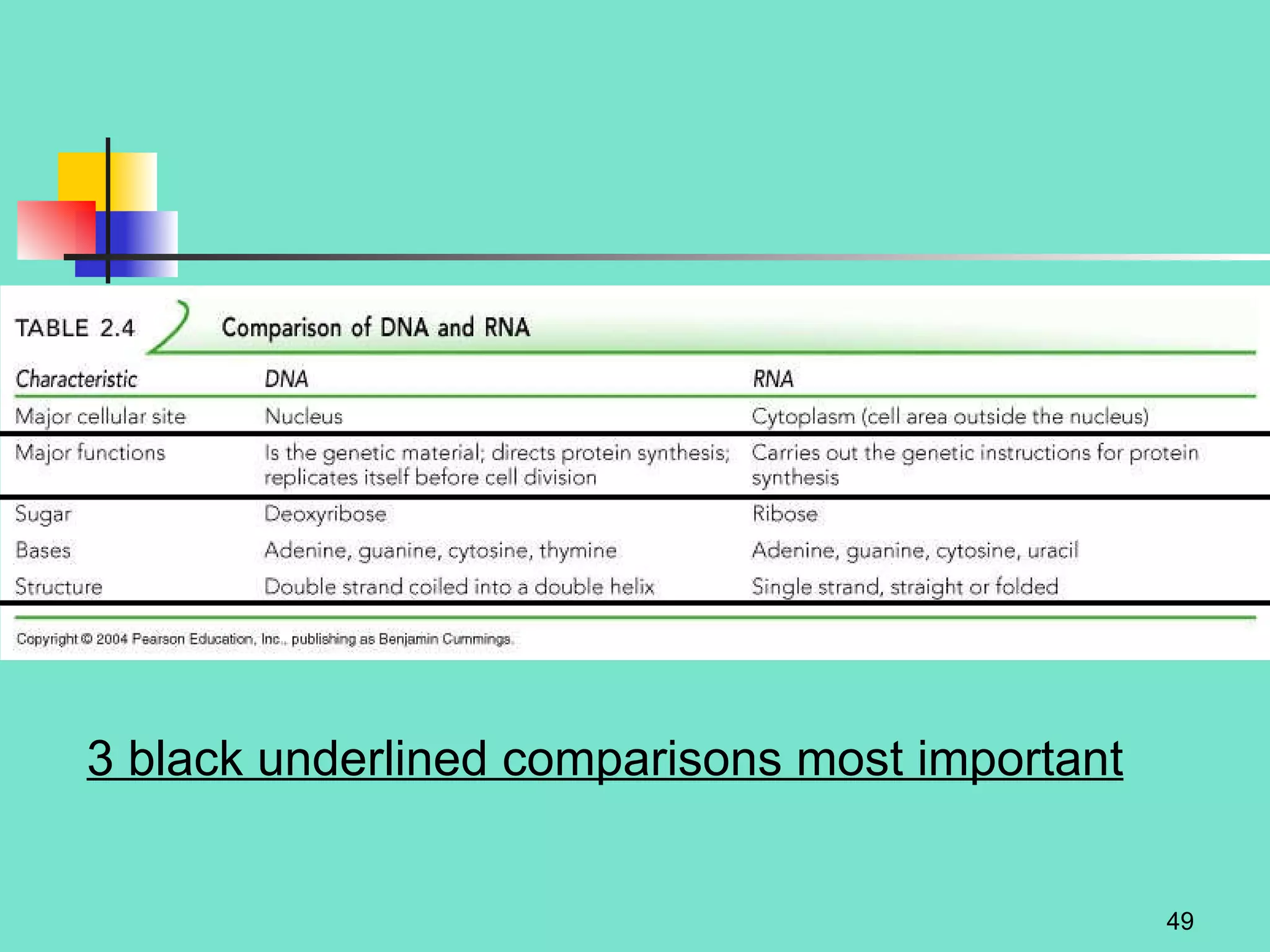
3. Explanations of organic compounds important for living things, including carbohydrates, proteins, lipids, and nucleic acids. It describes the monomers, polymers, and functions of each type of organic molecule.