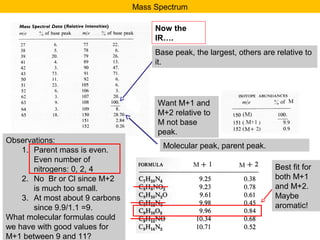
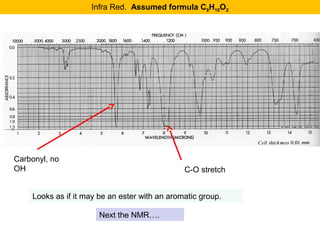
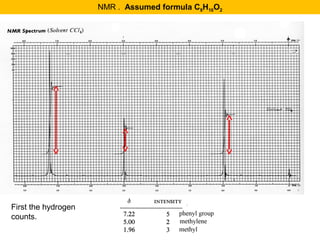
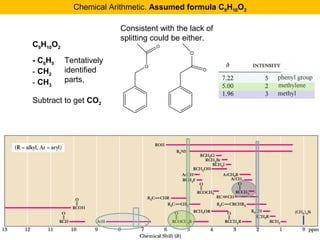
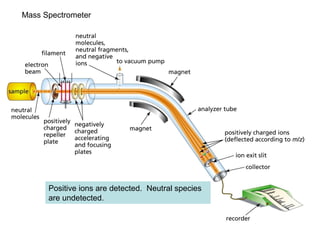
This document discusses techniques in mass spectrometry including:
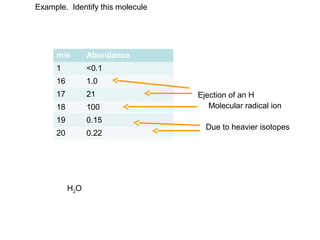
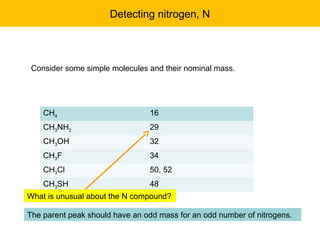
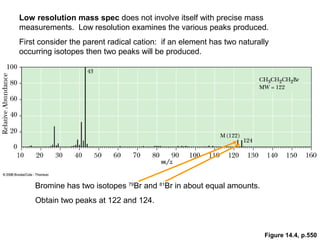
- The M+1 peak results from one atom being a heavier isotope by 1 mass unit. The M+2 peak can result from one atom being heavier by 2 units or two atoms each being heavier by 1 unit.
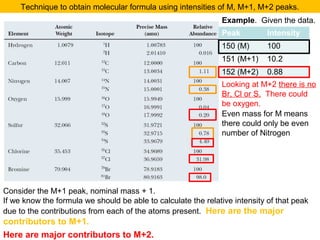
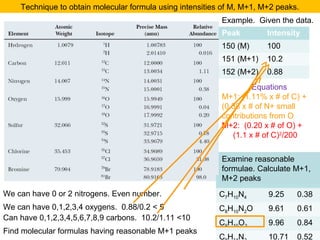
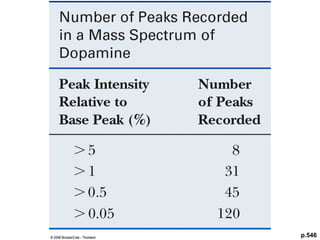
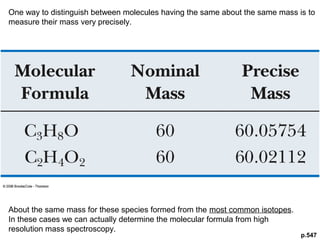
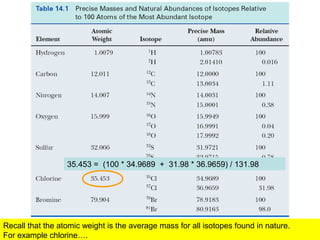
- Isotopic abundances can be used to calculate expected relative intensities of the M+1 and M+2 peaks and determine the molecular formula.
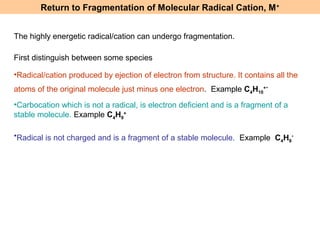
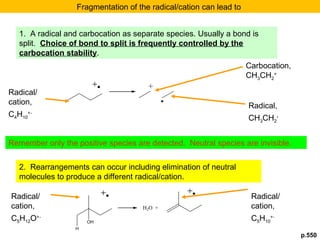
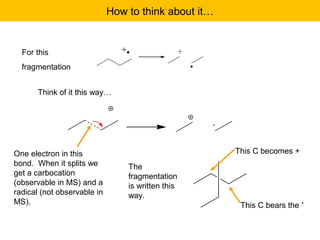
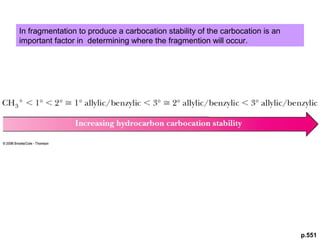
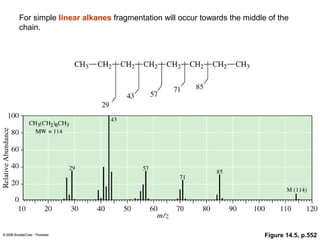
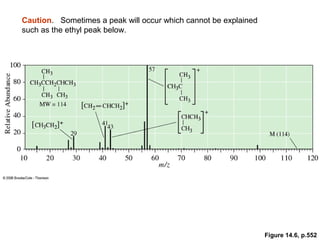
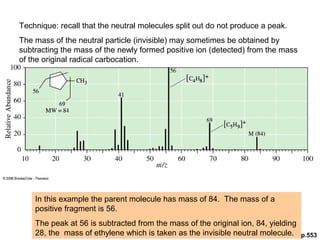
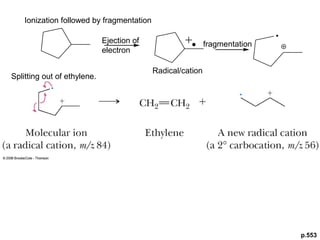
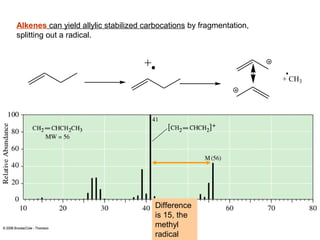
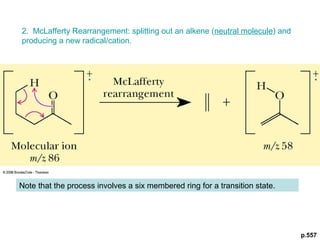
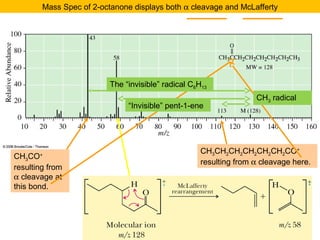
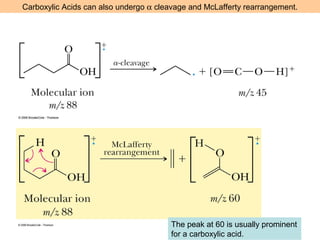
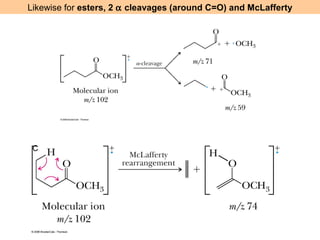
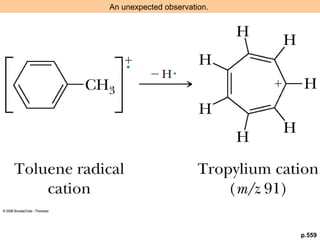
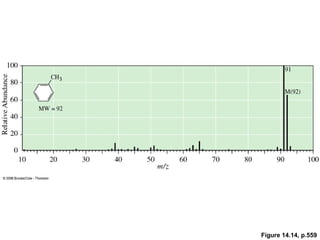
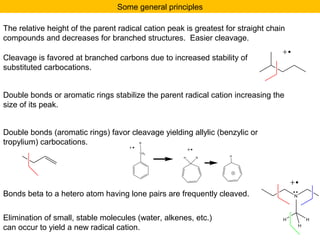
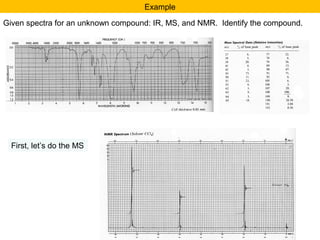
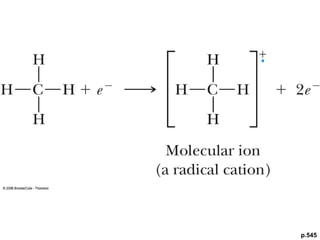
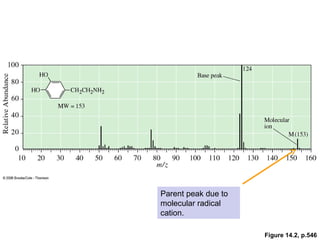
- Fragmentation of the molecular radical cation can yield carbocations and radicals. Bond cleavage is influenced by carbocation stability.
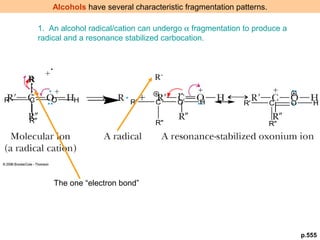
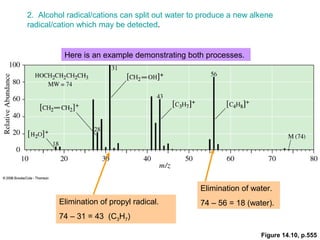
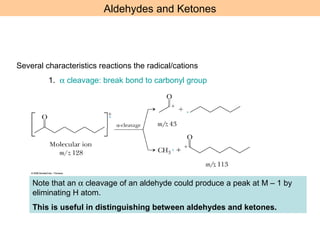
- Characteristic fragmentation patterns exist for functional groups like alcohols, aldehydes and ketones, carboxylic acids, and esters.

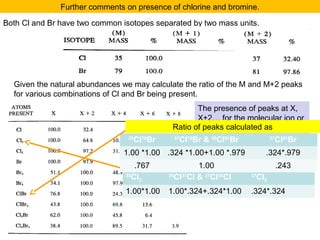


![M+2 peak, contributions from one atom and two atoms.
Recap:
The M+1 peak has contributions from one atom being a heavier isotope by 1.
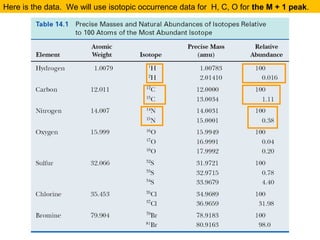
(M+1)/M = ca. 1.1% * no. of C atoms + 0.36% * no. of N atoms
The M+2 peak can have contributions from two sources
•One atom being a heavier isotope by 2. Mainly O (excluding S, Cl and Br)
•Two atoms being heavier by 1 each. Mainly C atoms.
(M+2)/M = ca. (0.20% * no. of O atoms) + (1.1 * no. of C atoms)2/200%
Example 1: C5H5N
[(A + 1)+]/[A+] = 5 x 1.1% + 1 x 0.36% =
5.9%
[(A + 2)+]/[A+] = 5.52/200 % = 0.15%
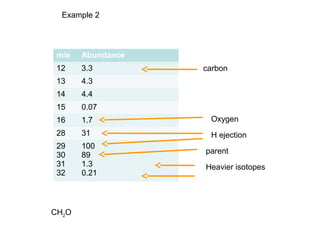
Example 2: C7H5O
[(A + 1)+]/[A+] = 7 x 1.1% = 7.7%
[(A + 2)+]/[A+] = 7.72/200 % + 0.20% = 0.50%](https://image.slidesharecdn.com/chap14massspec-140928235902-phpapp02/85/Chap-14-mass-spec-14-320.jpg)