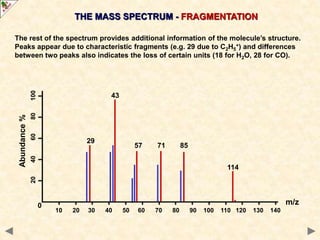
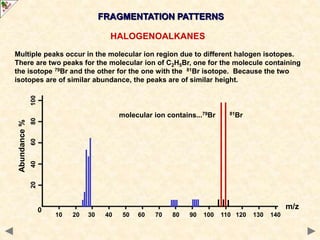
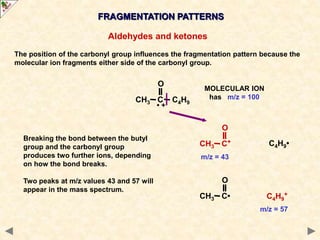
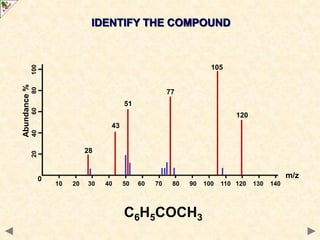
This document is a guide on mass spectrometry for A-level chemistry students. It discusses the basic components and principles of mass spectrometry, including ionization, fragmentation, and molecular mass determination. It provides examples of mass spectra for different organic functional groups like alkanes, halogenoalkanes, aldehydes, and ketones. It explains how to interpret mass spectra and identify compounds based on their fragmentation patterns. Students are tested on their understanding and ability to interpret mass spectra through example problems.