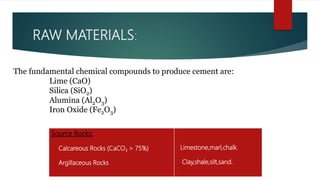
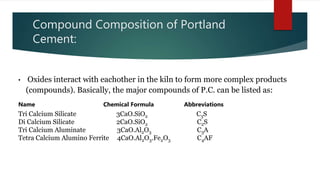
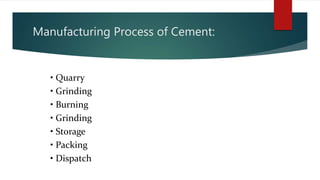
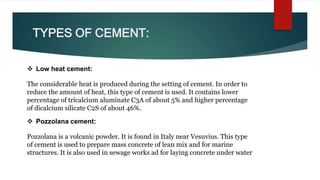
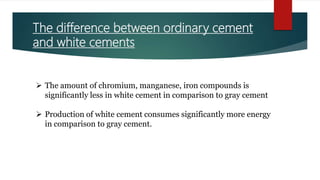
Cement is a hydraulic compound essential for construction, primarily used in making concrete, mortar, and plaster. It is derived from calcined lime and clay with a history tracing back to ancient uses, while the modern Portland cement was invented in 1824. Pakistan has ample limestone and clay reserves supporting a growing cement production industry, currently producing over 19.5 million tonnes annually to meet a demand of around 9.5 million tonnes.