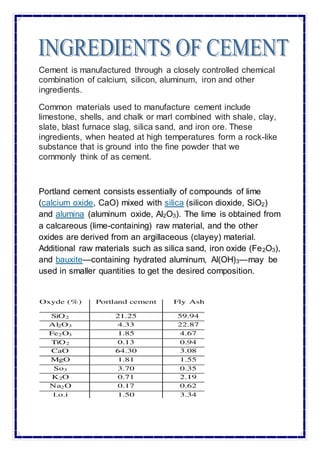
The document discusses the history and development of Portland cement. It notes that John Smeaton discovered that a mixture of lime, clay and iron slag produced a mortar that hardened under water in 1759. Joseph Aspdin patented "Portland Cement" in 1824, calling it such because the concrete resembled Portland stone. Isaac Johnson further developed modern Portland cement in 1845 by firing chalk and clay at higher temperatures. Key later developments included the use of rotary kilns, addition of gypsum to control setting, and ball mills for grinding. The document then discusses the chemical and physical properties of cement, different cement grades, and testing and storage of cement.