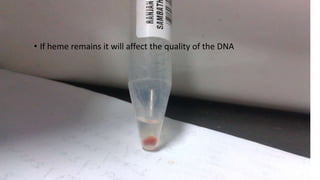
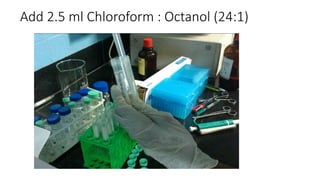
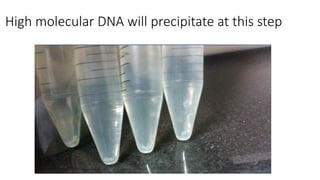
The document details a protocol for DNA extraction using the phenol-chloroform method, outlining the materials and step-by-step procedures for handling blood samples and isolating DNA. It specifies necessary reagents, equipment, and various steps including lysis, centrifugation, and purification processes over two days. The final goal is to obtain high-quality DNA for further analysis such as quantification and genotyping.