1. Celiac disease is an immune-mediated disorder triggered by gluten in genetically susceptible individuals, characterized by clinical manifestations, anti-tissue transglutaminase antibodies, and enteropathy.
2. It has a prevalence of 1 in 300 to 1 in 80 children between ages 2.5-15 years. Increased risk is seen in those with type 1 diabetes, autoimmune liver/thyroid diseases, IgA deficiency, or having a first-degree relative with celiac disease.
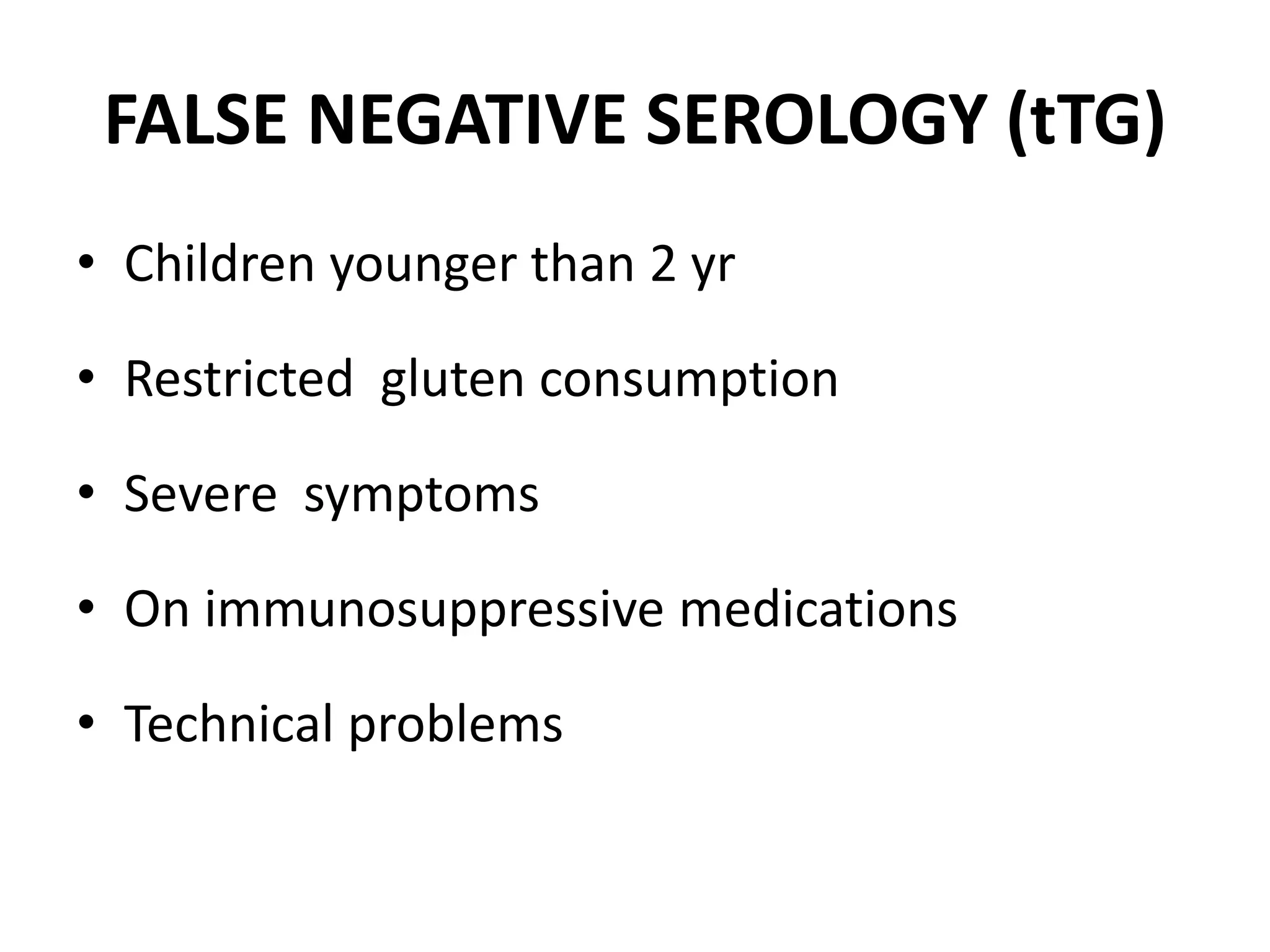
3. Diagnosis involves testing for anti-tTG and EMA antibodies, followed by biopsy of the duodenum if antibodies are positive. A gluten-free diet is the primary treatment if celiac disease is confirmed