Embed presentation
Downloaded 42 times




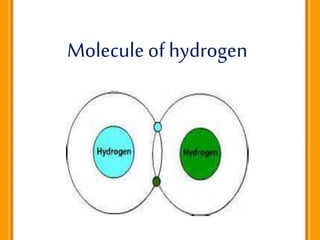



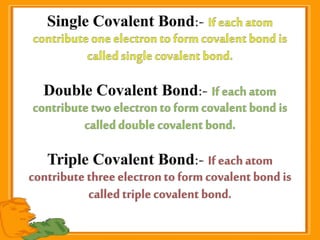
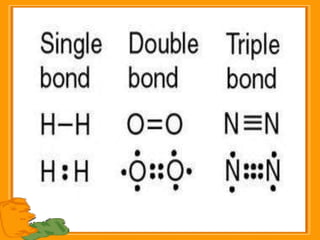

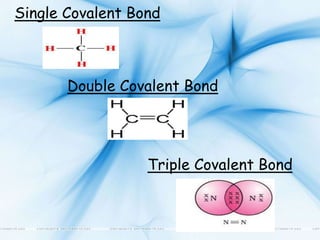




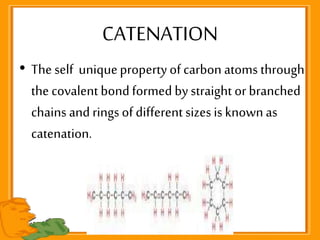




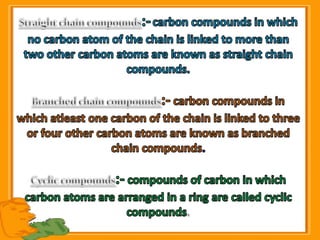
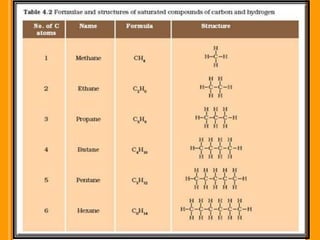




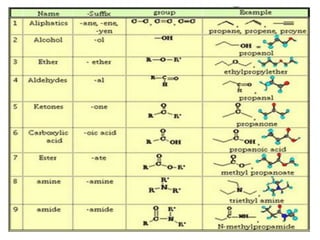


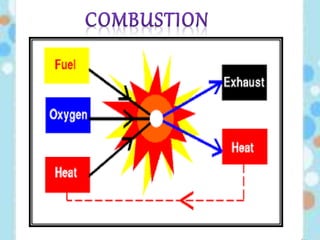

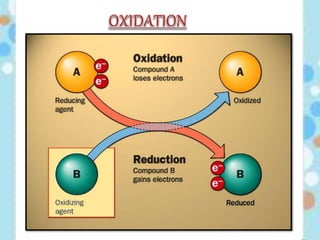

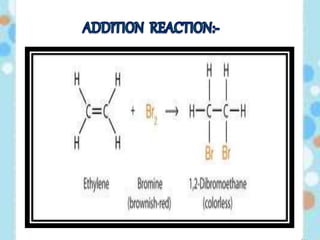

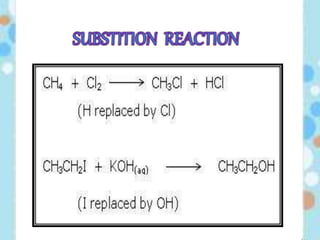

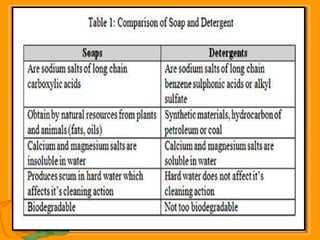

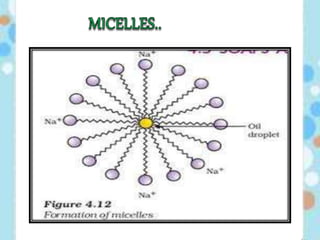


Carbon exists in several allotropic forms and can form single, double, or triple covalent bonds with other carbon atoms. It has a versatile nature due to its ability to catenate, or form straight or branched chains or rings of different sizes through covalent bonds. Carbon compounds are classified as saturated if they only contain single bonds, or unsaturated if they contain one or more double or triple bonds.










































