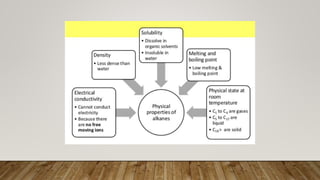
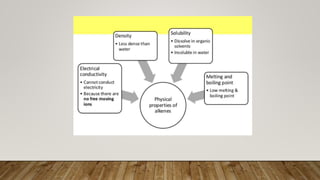
Carbon compounds can be organic or inorganic. Organic compounds contain carbon and hydrogen, forming the building blocks of life like proteins, lipids, carbohydrates and nucleic acids. Inorganic carbon compounds do not necessarily contain hydrogen, and include carbon dioxide and iron oxide. While most carbon compounds are organic, some like carbon dioxide are classified as inorganic due to weaker carbon bonds.