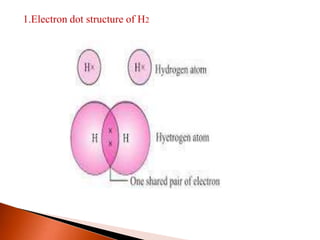
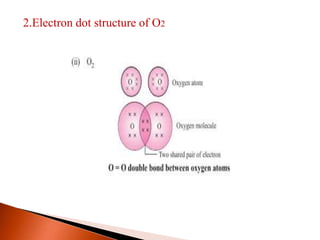
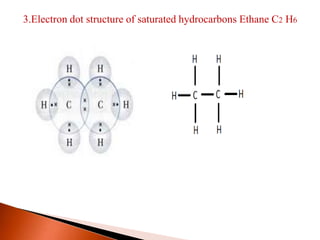
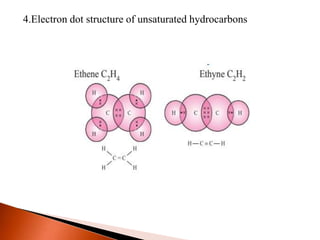
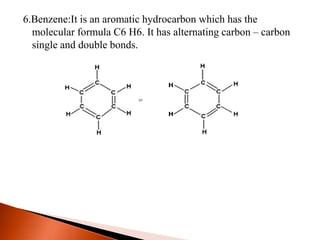
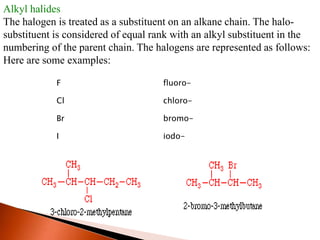
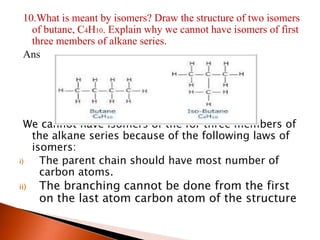
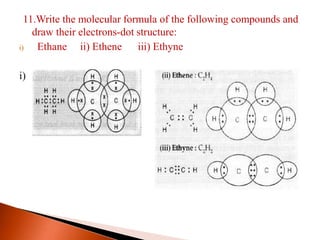
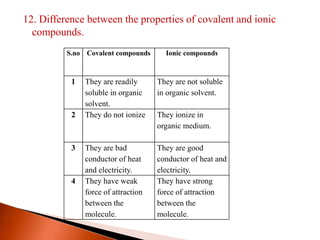
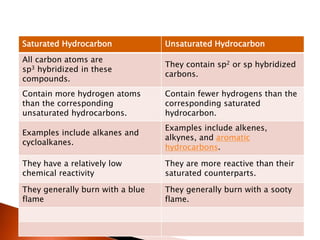
This document provides information about carbon and its compounds. It discusses electron dot structures of various molecules like H2, O2, ethane and unsaturated hydrocarbons. It also describes cyclic/closed chain hydrocarbons and aromatic hydrocarbons like benzene. The document outlines IUPAC naming rules for hydrocarbons and different formula types. It provides examples of alkenes, alkynes and their naming conventions. Key differences between properties of covalent and ionic compounds are highlighted.