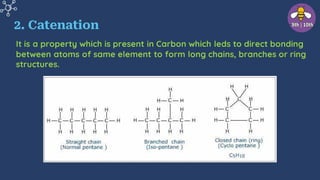
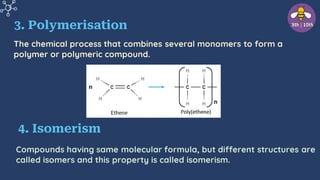
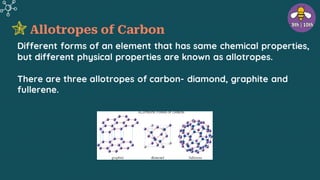
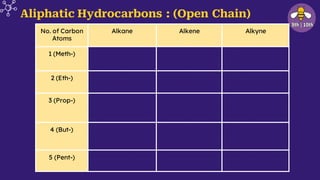
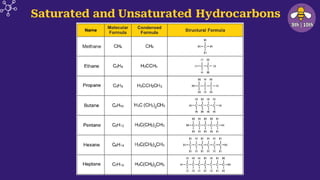
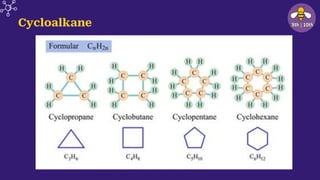
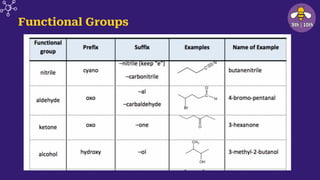
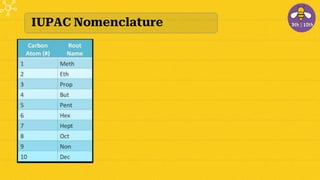
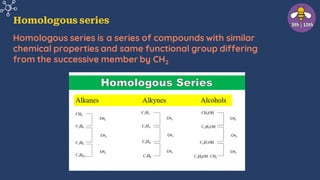
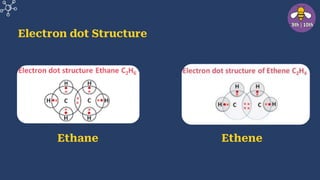
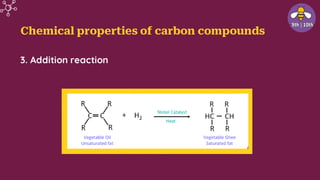
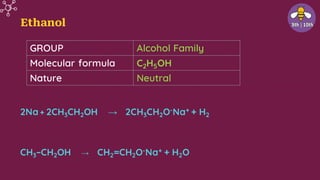
The document provides an overview of carbon and its compounds, highlighting carbon's unique properties such as tetravalency, catenation, polymerization, and isomerism. It discusses the various allotropes of carbon, including diamond, graphite, and fullerene, and the significance of carbon-containing compounds, particularly hydrocarbons. Additionally, it covers chemical reactions involving carbon compounds and differentiates between soaps and detergents.