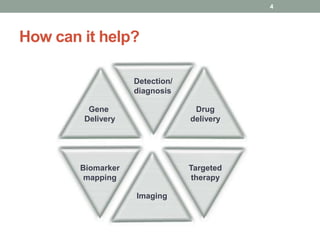
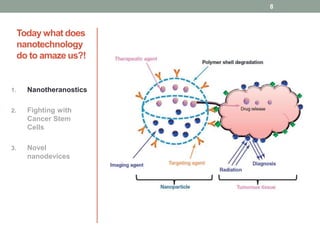
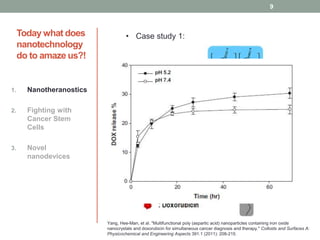
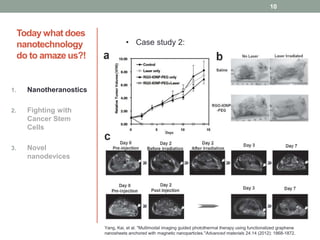
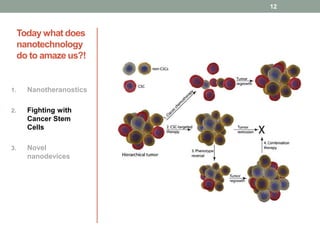
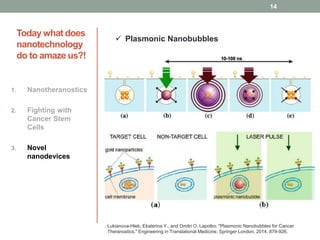
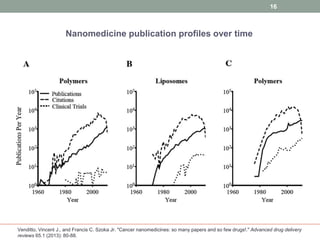
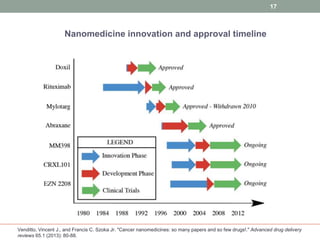
The document discusses the promise of nanotechnology for cancer treatment and diagnosis. It outlines how nanotechnology can help with detection, drug delivery, targeted therapy, imaging, and gene delivery. Some ways nanotechnology is currently being used include nanotheranostics, which allow simultaneous diagnosis and treatment, targeting cancer stem cells, and novel nanodevices like plasmonic nanobubbles. While nanotechnology shows potential, challenges remain around toxicity, costs, and translating research findings into approved drugs. The document calls for biotechnologists to provide ideas to further advance the field of cancer nanotechnology.