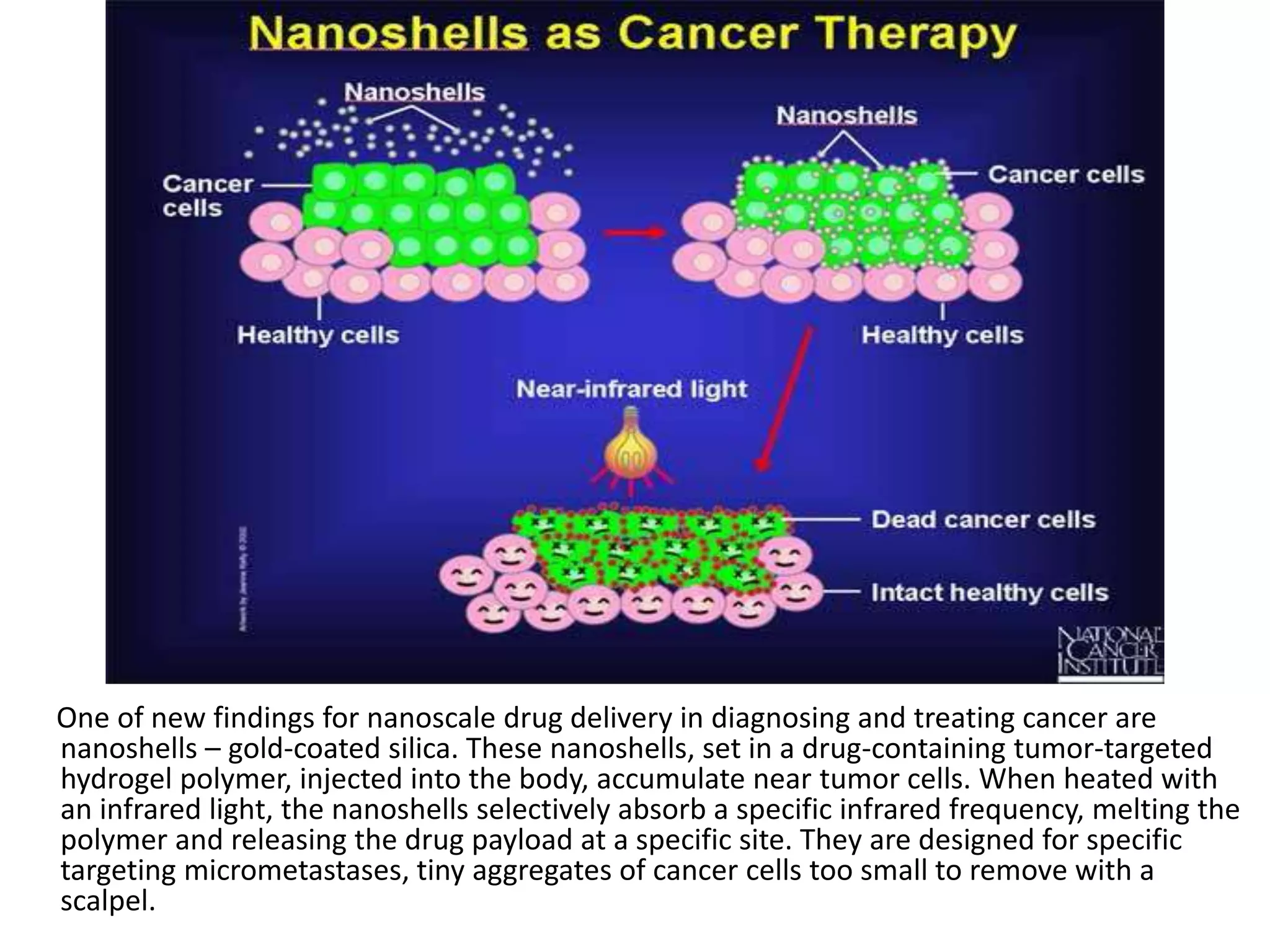
This document discusses the use of nanoparticles in cancer diagnosis and treatment. It introduces several types of nanoparticles that can be used, including nanoshells, dendrimers, quantum dots, superparamagnetic nanoparticles, nanowires, nanodiamonds, and nanosponges. Nanoshells and dendrimers are highlighted as promising for targeted drug delivery. The document also discusses magnetic resonance imaging contrast agents, including both paramagnetic gadolinium agents and superparamagnetic iron oxide nanoparticles, which can enhance MRI images and improve cancer diagnosis.
















![Superparamagnetic contrast agents
• Several classes of magnetic iron oxide nanoparticles, also
known as superparamagnetic IONPs (SPIO) and ultrasmall
SPIO (USPIO) developed in 1980s, has been approved by FDA
(e.g., Feridex) for clinical applications with capabilities of
traditional “blood pool” agents.[20, 21] The important
properties of cell phagocytosis of magnetic nanoparticles has
expanded the applications of contrast enhanced MRI beyond
the vascular and tissue morphology imaging, enabling many
novel applications of magnetic IONPs for MRI diagnosis of
liver diseases, cancer metastasis to lymph nodes, and in vivo
tracking of implanted cell and grafts with MRI.](https://image.slidesharecdn.com/kdspharmaceutical-161207101857/75/NANOPARTICLES-IN-CANCER-DIAGNOSIS-AND-TREATMENT-18-2048.jpg)

