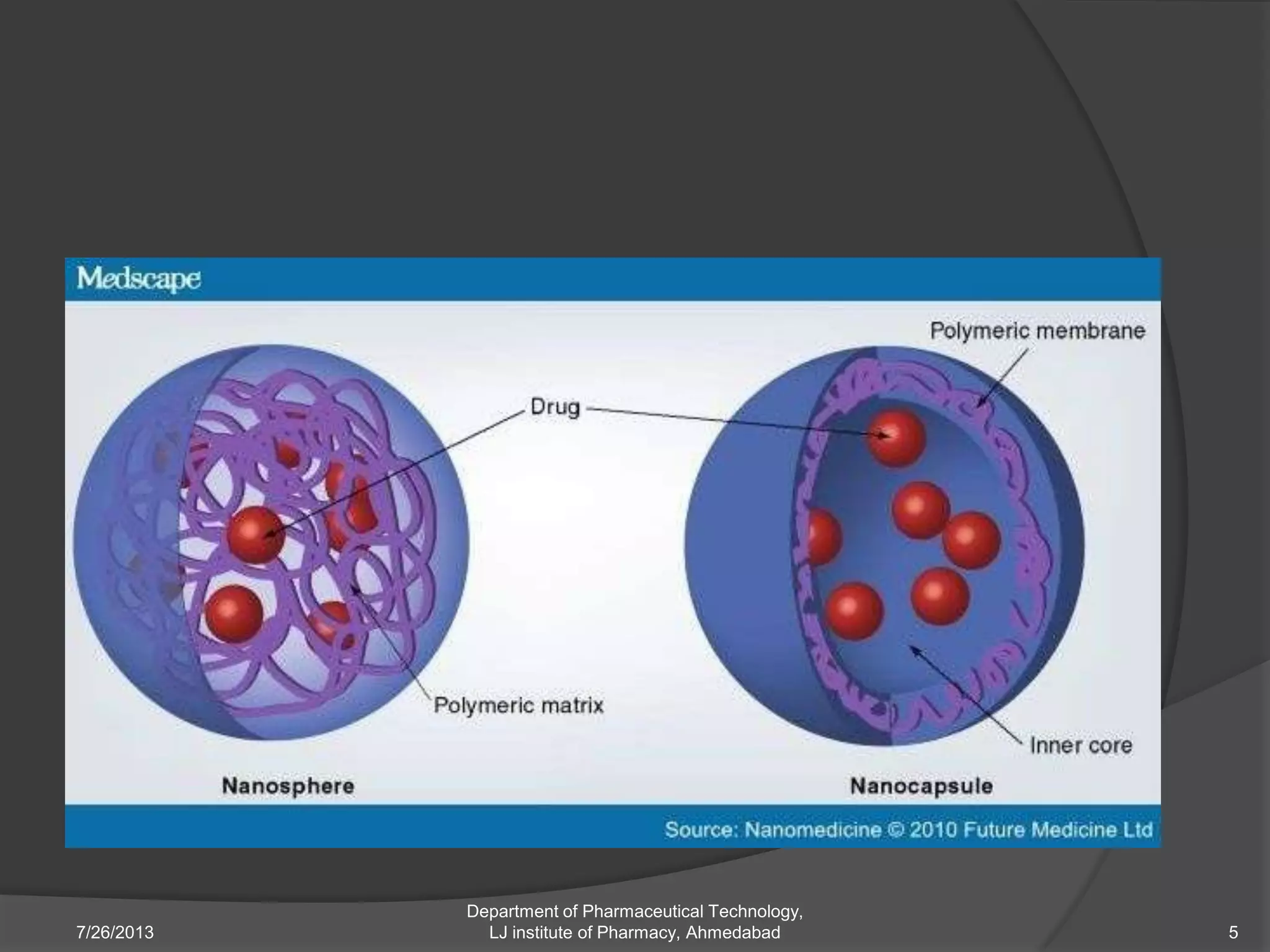
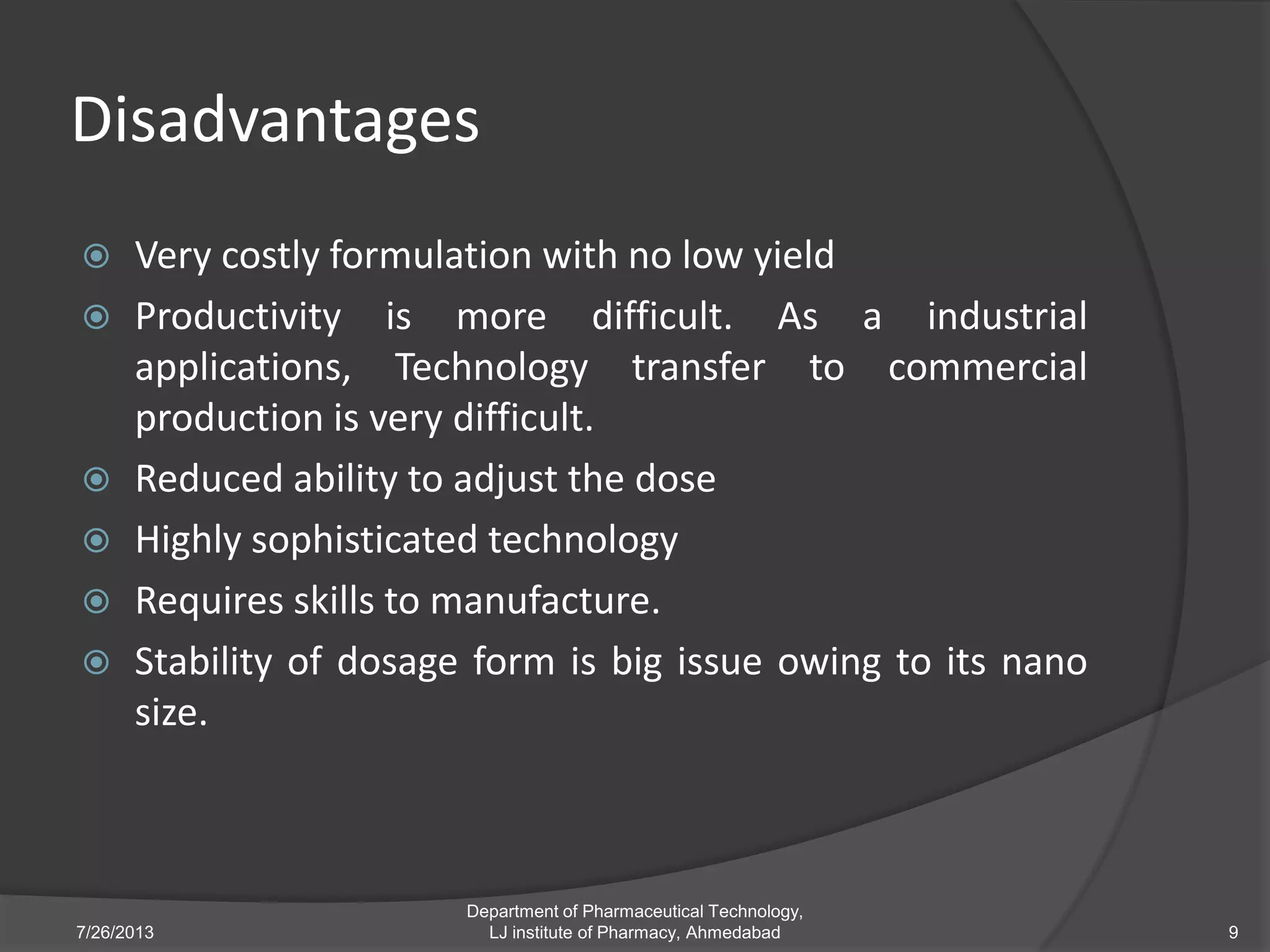
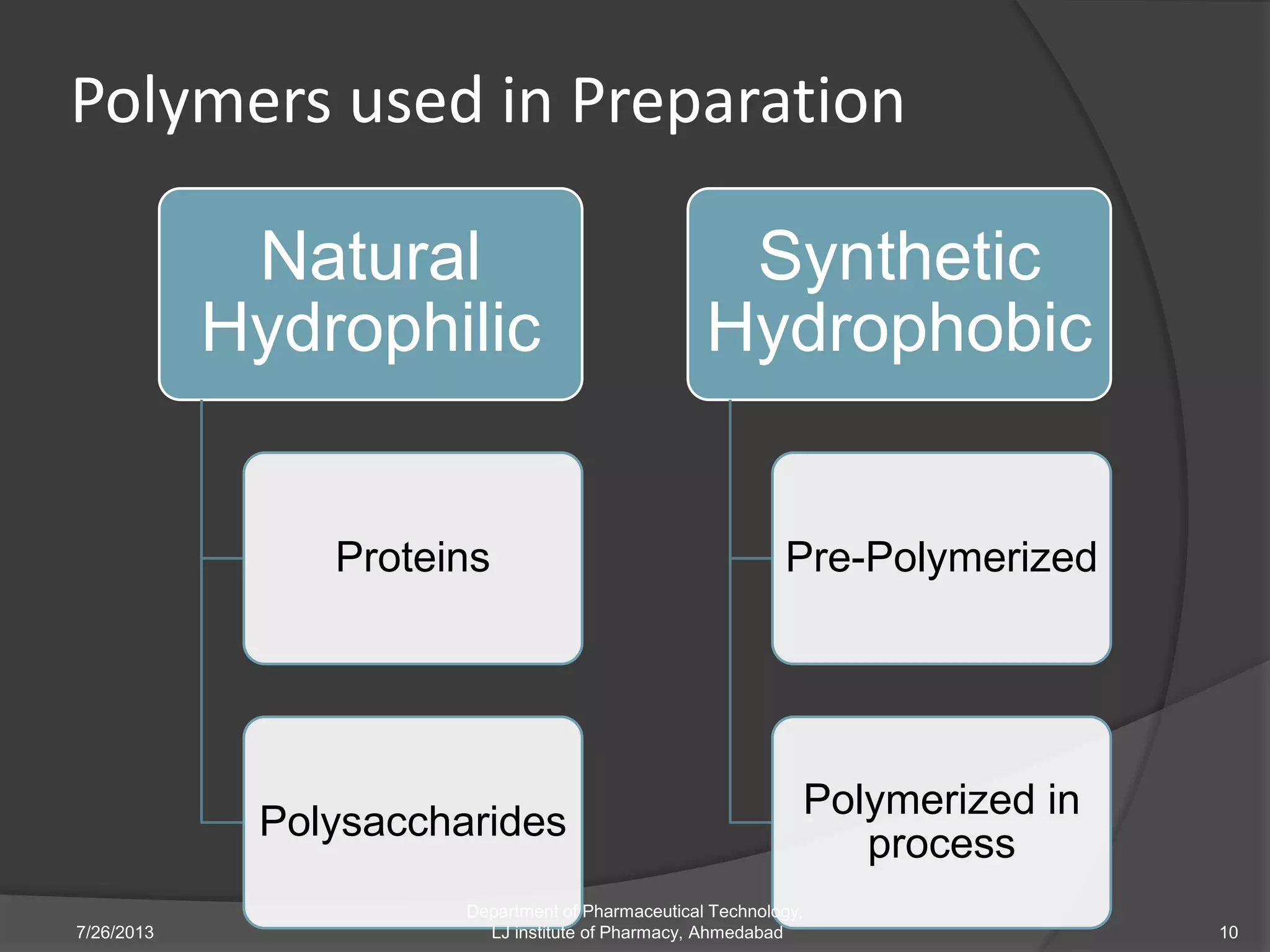
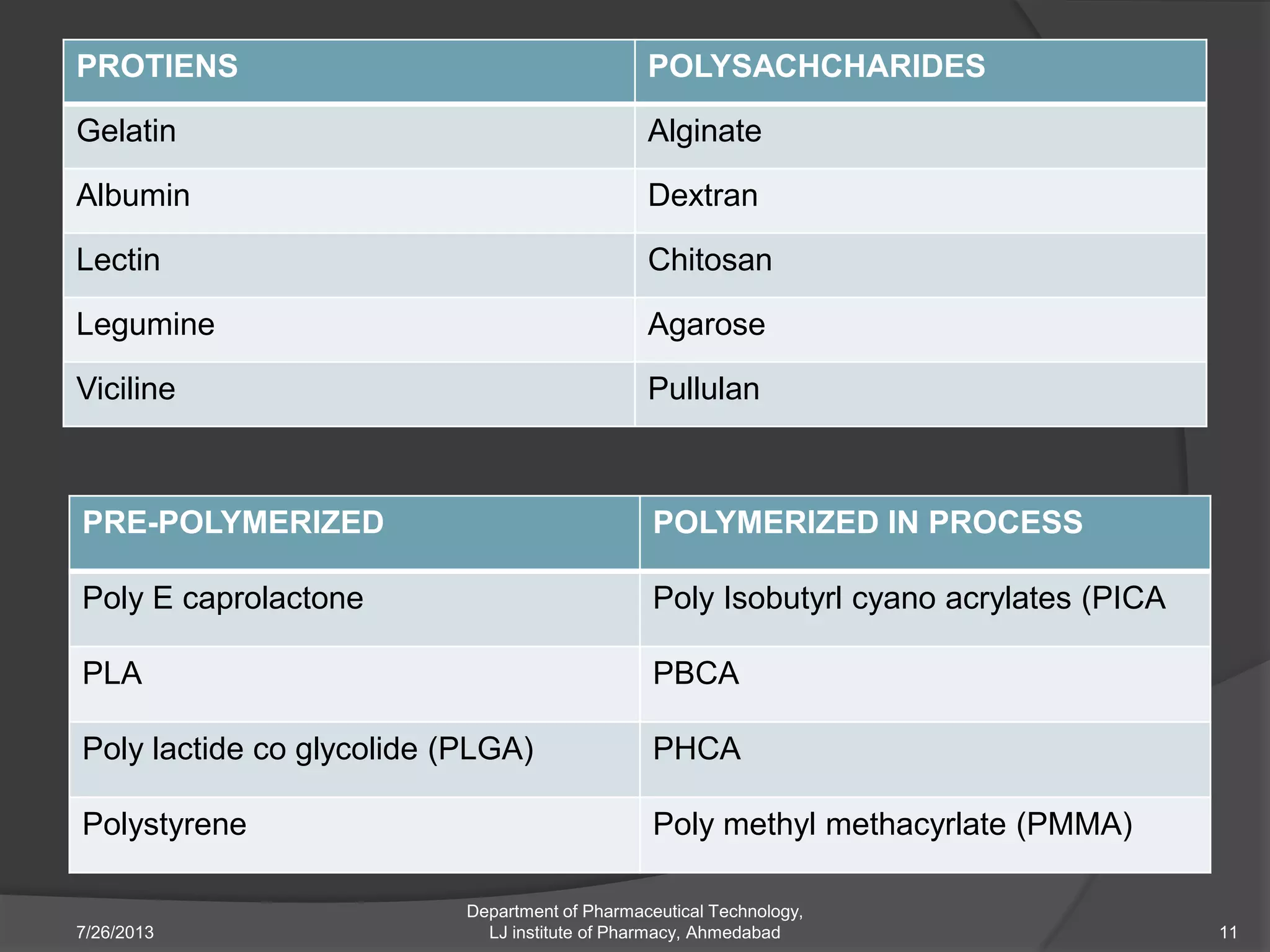
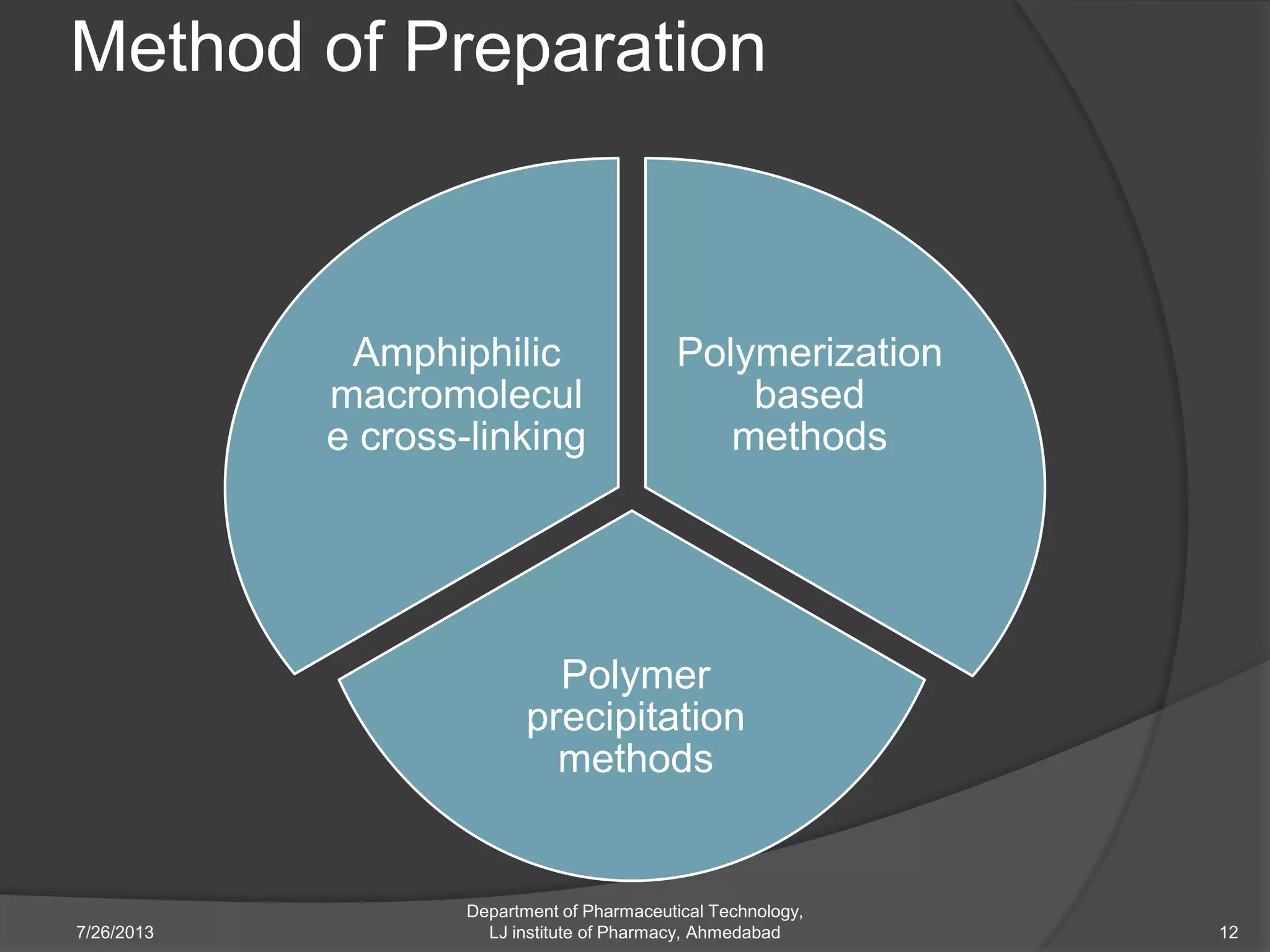
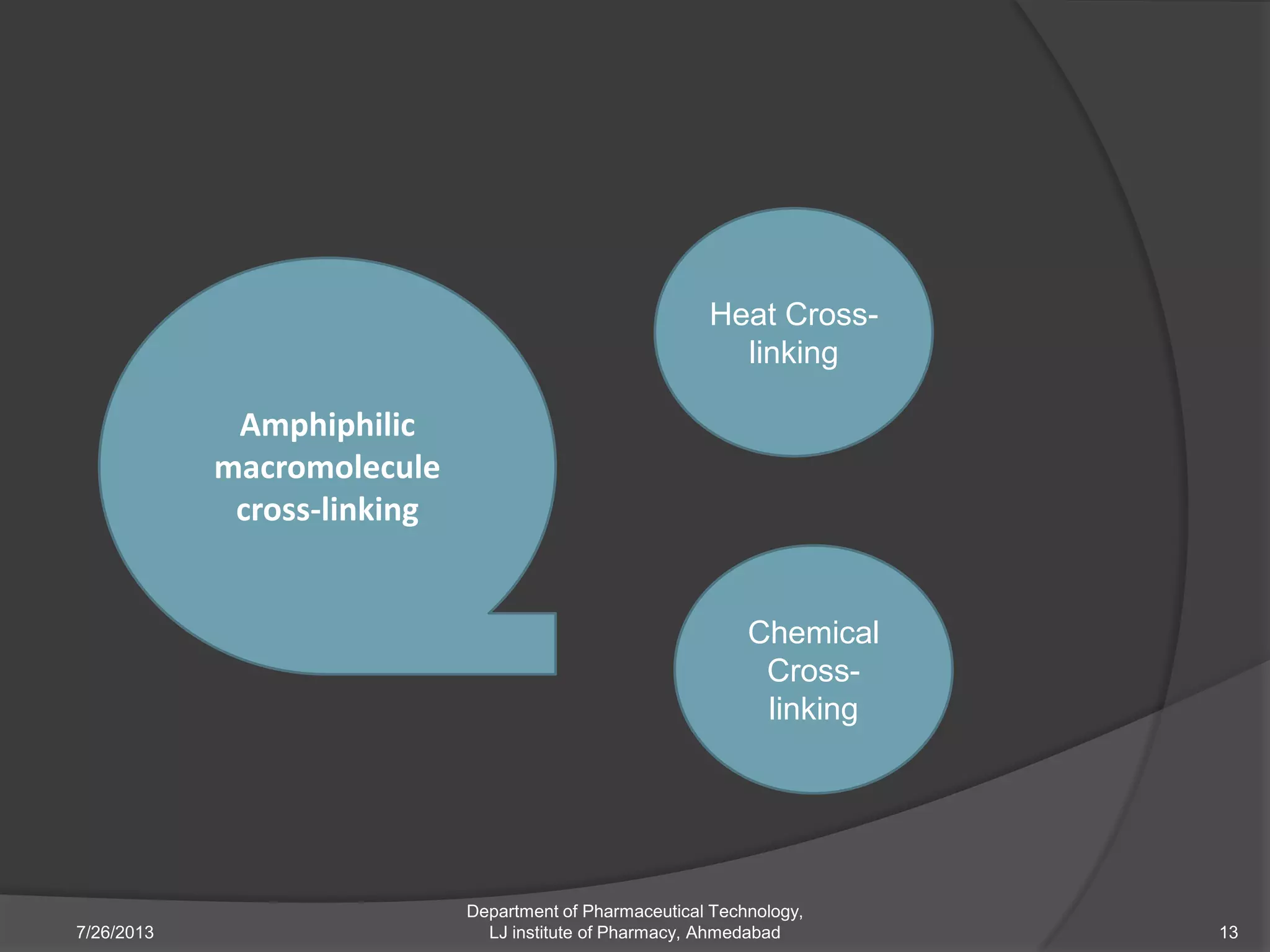
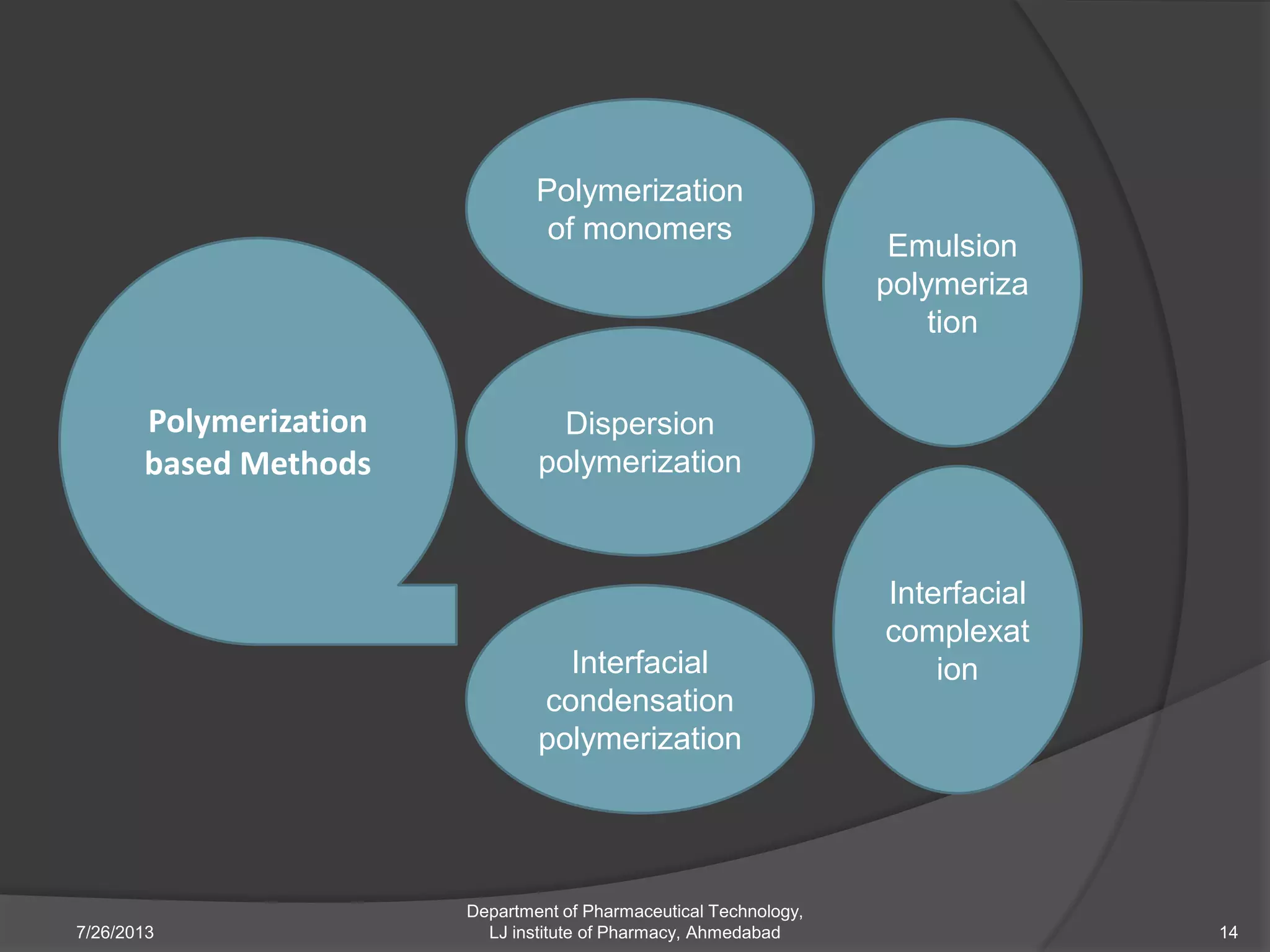
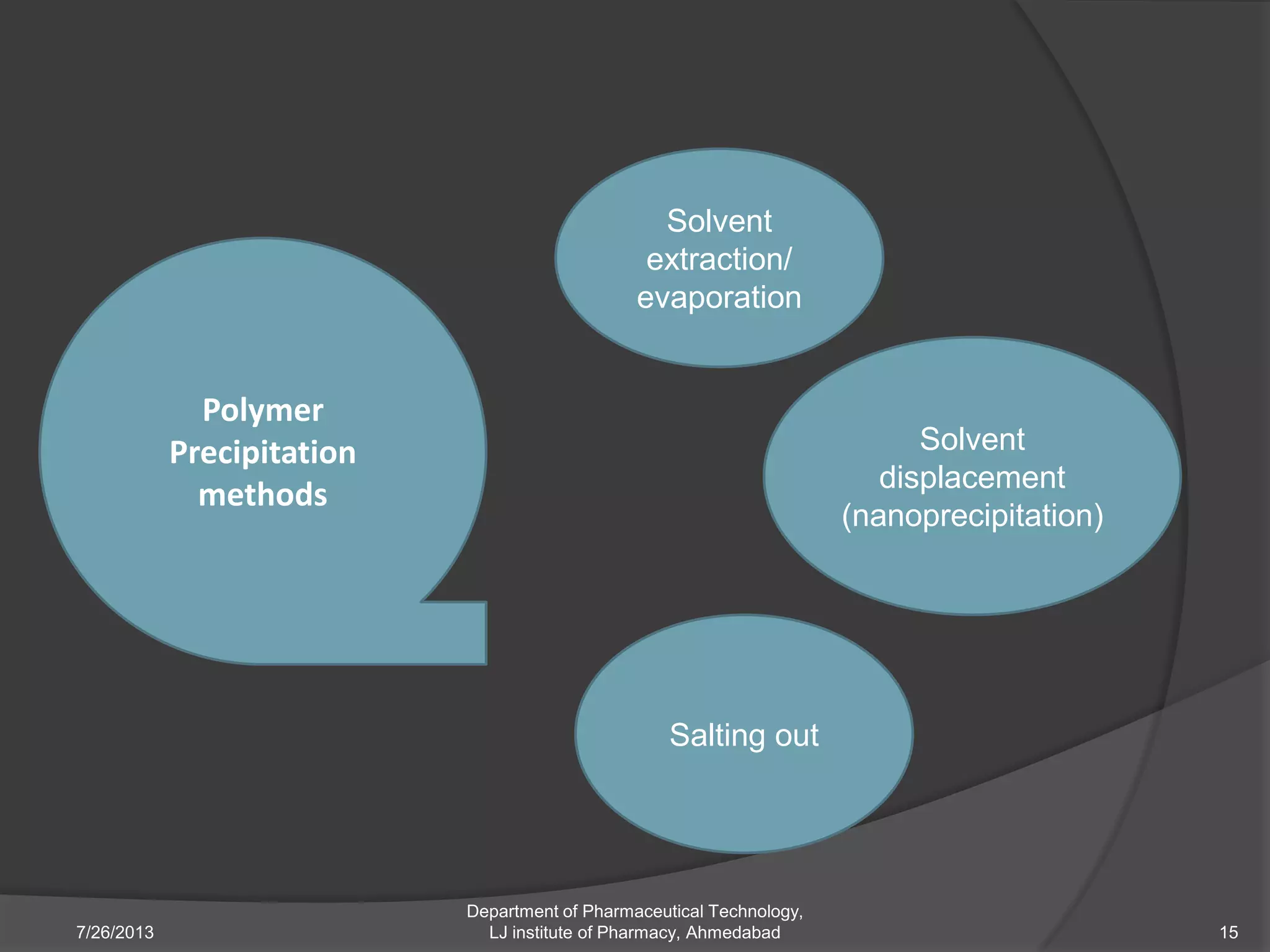
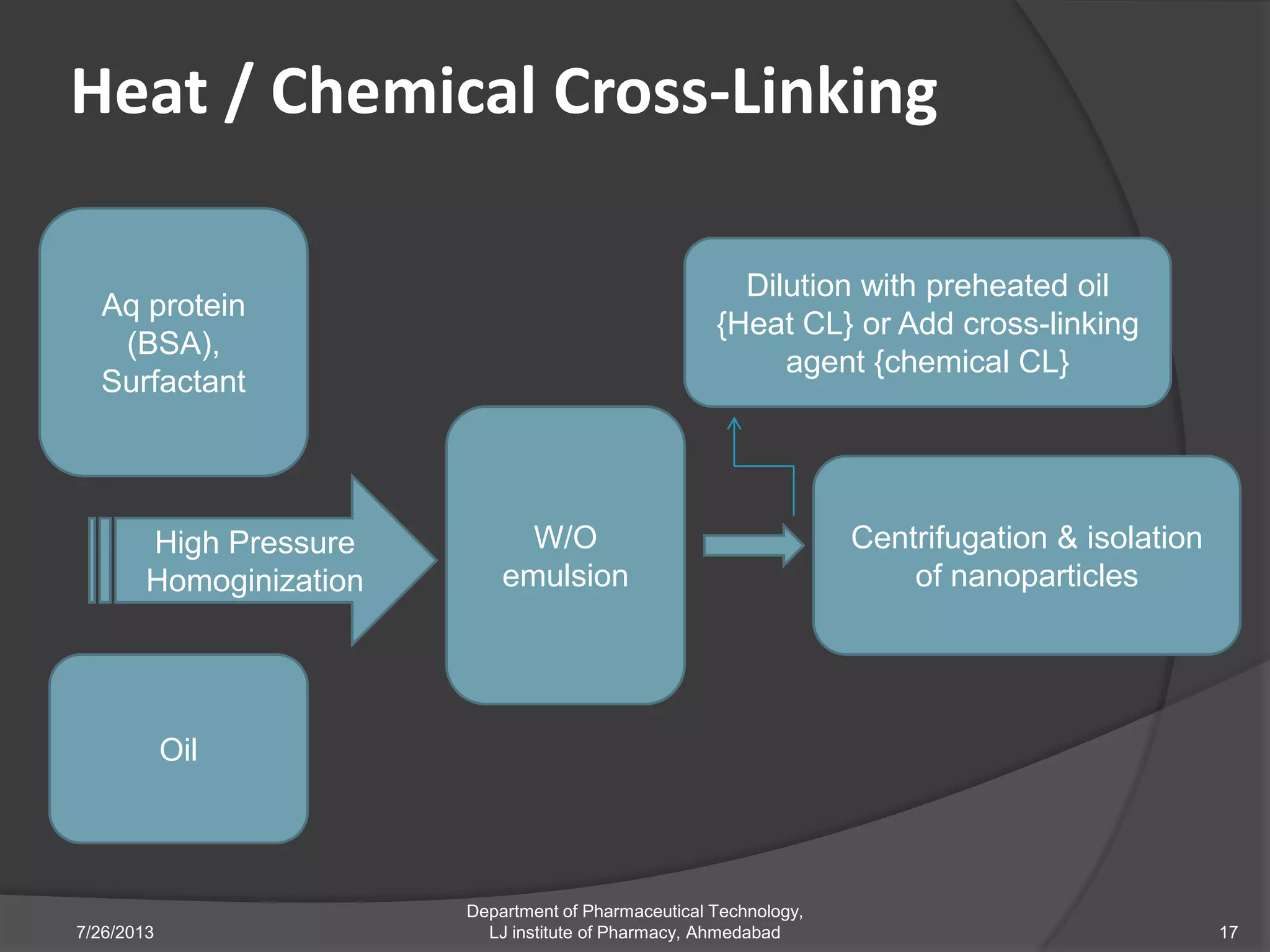
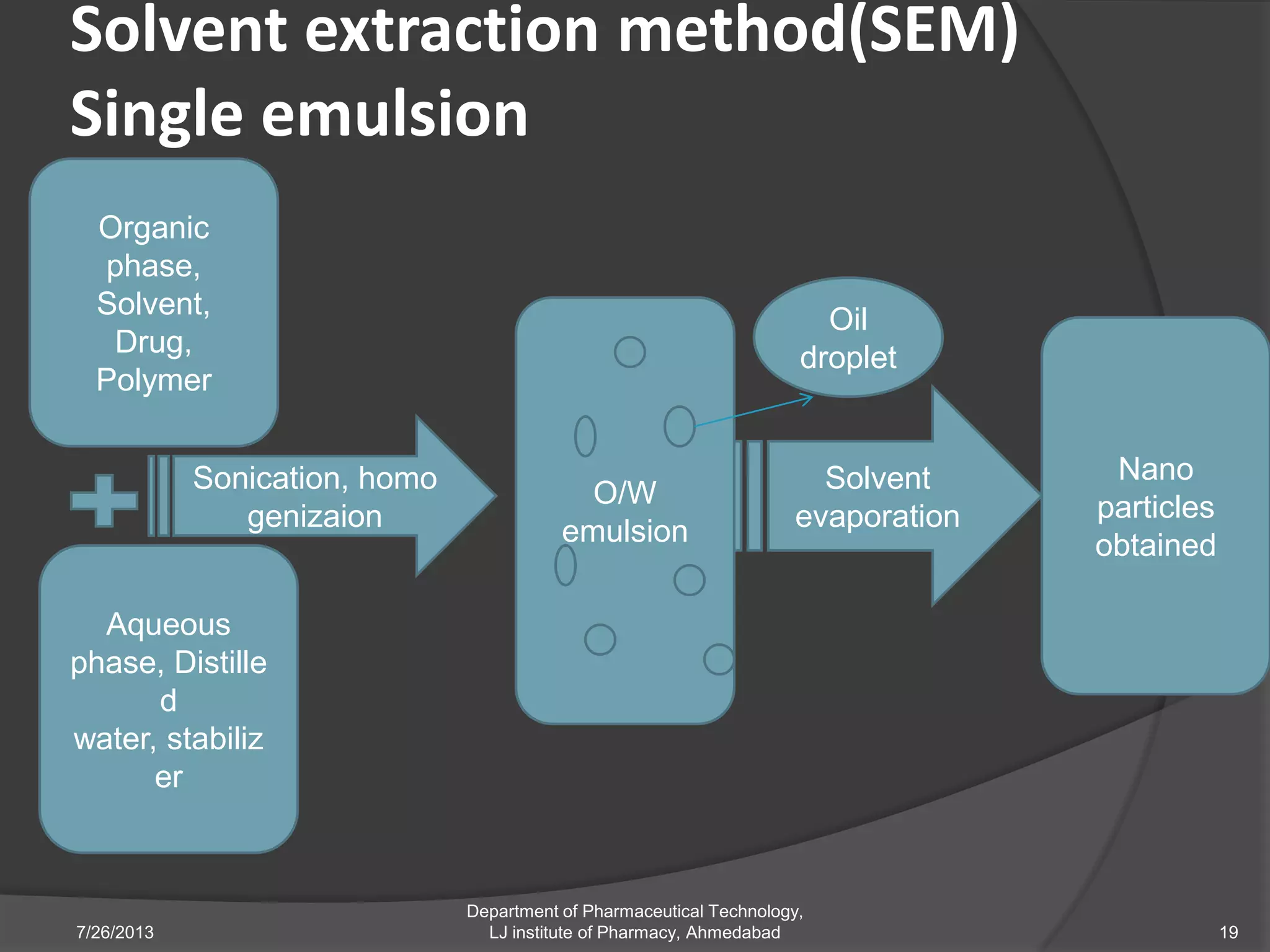
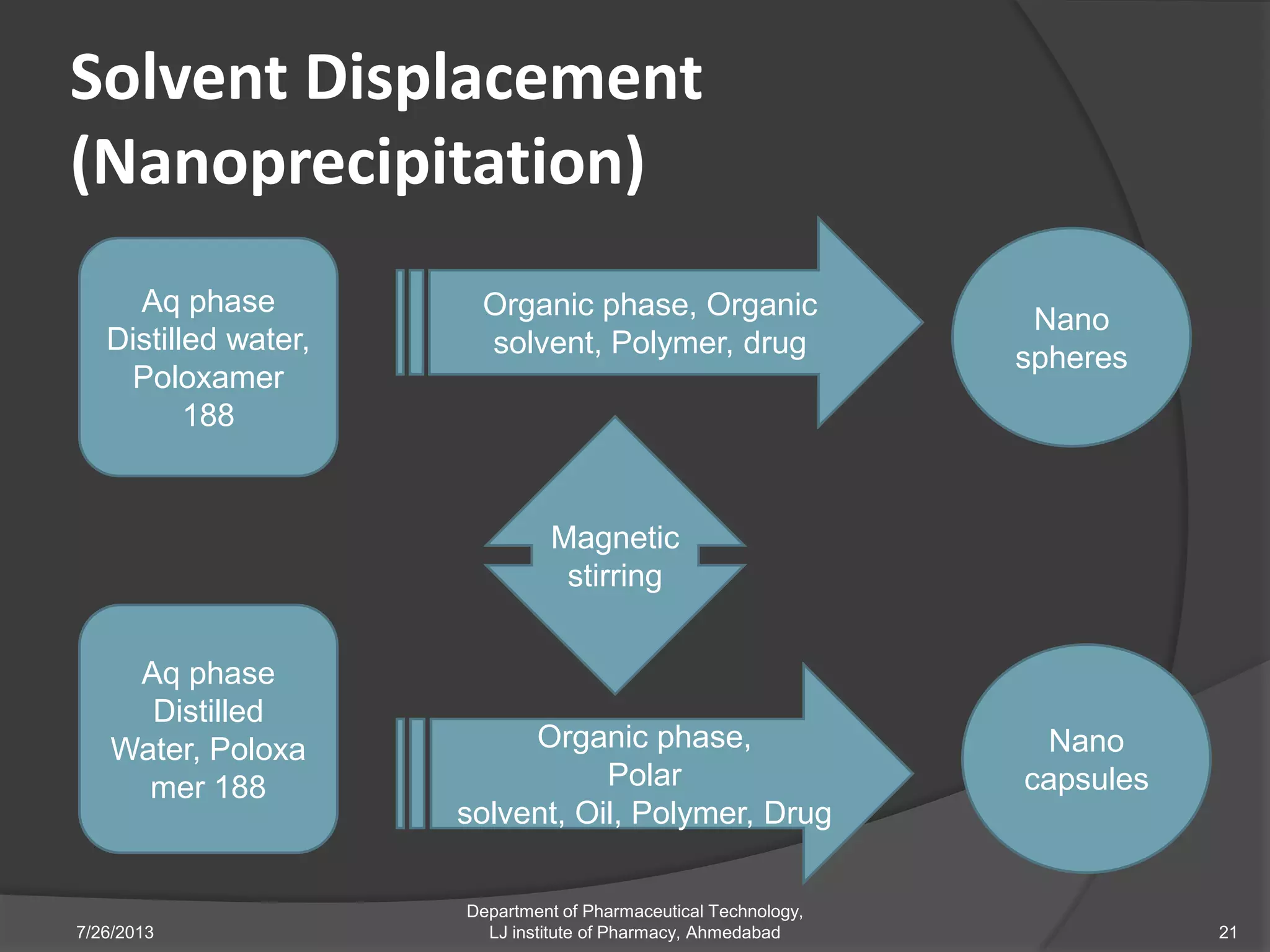
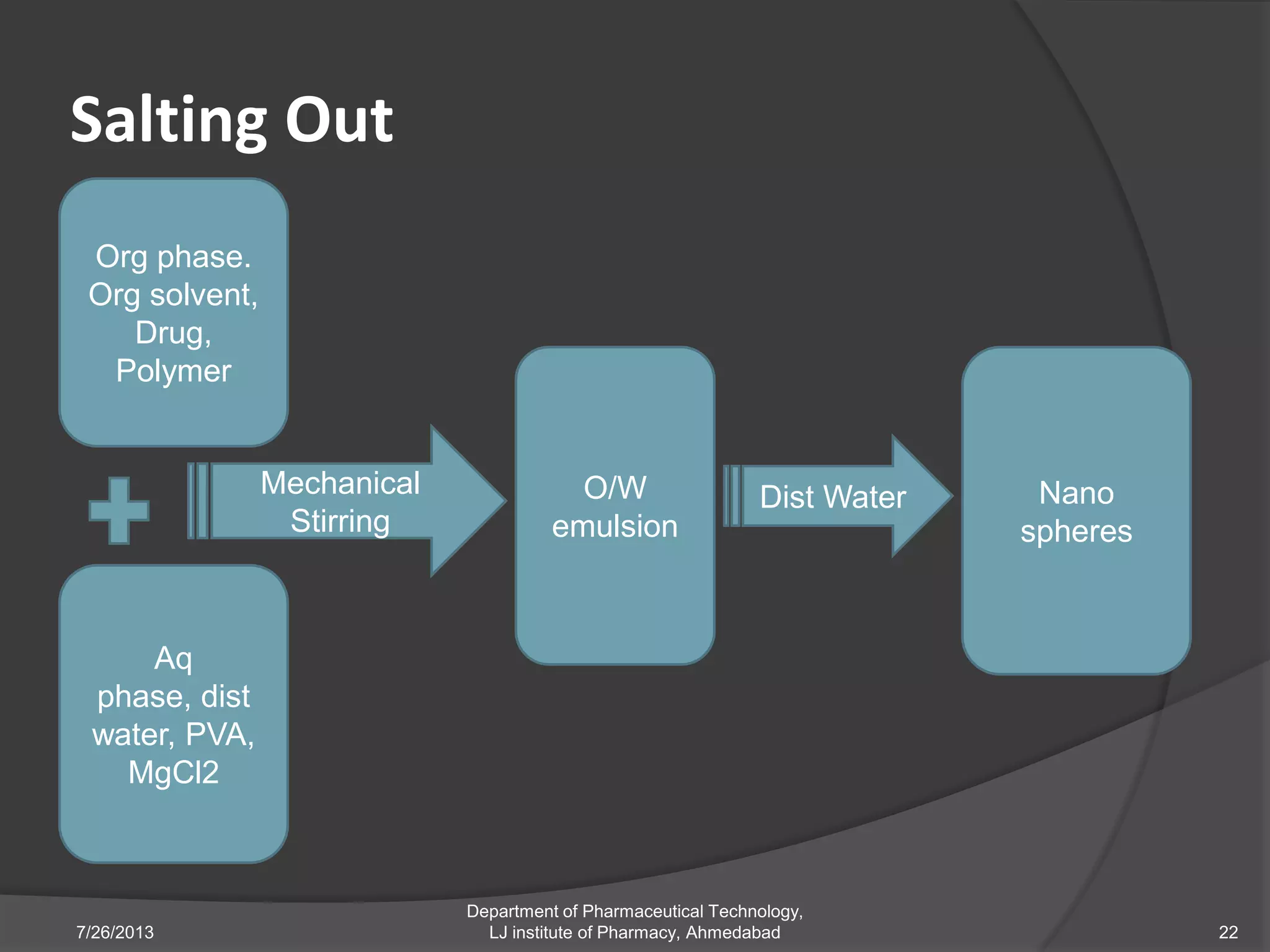
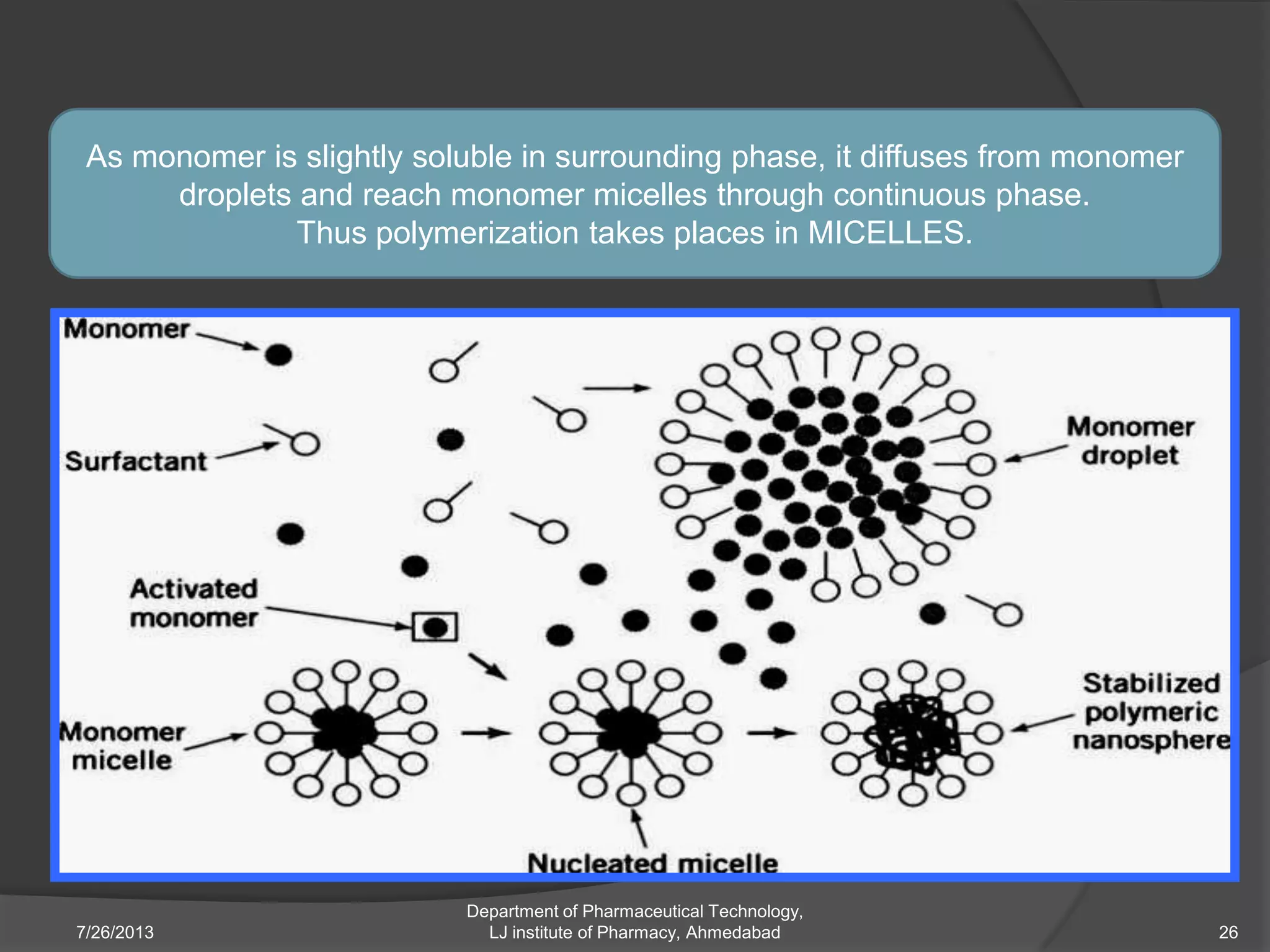
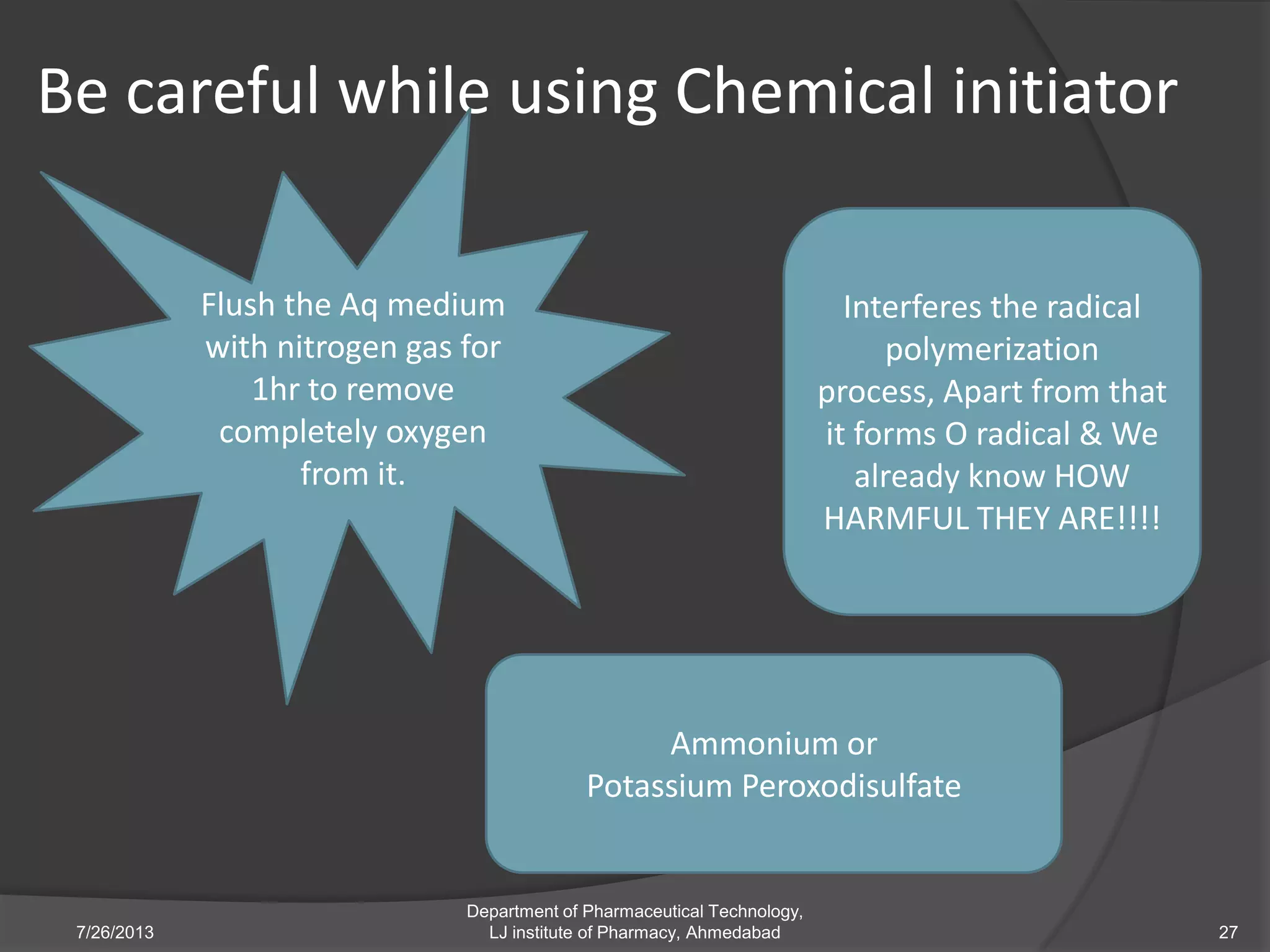
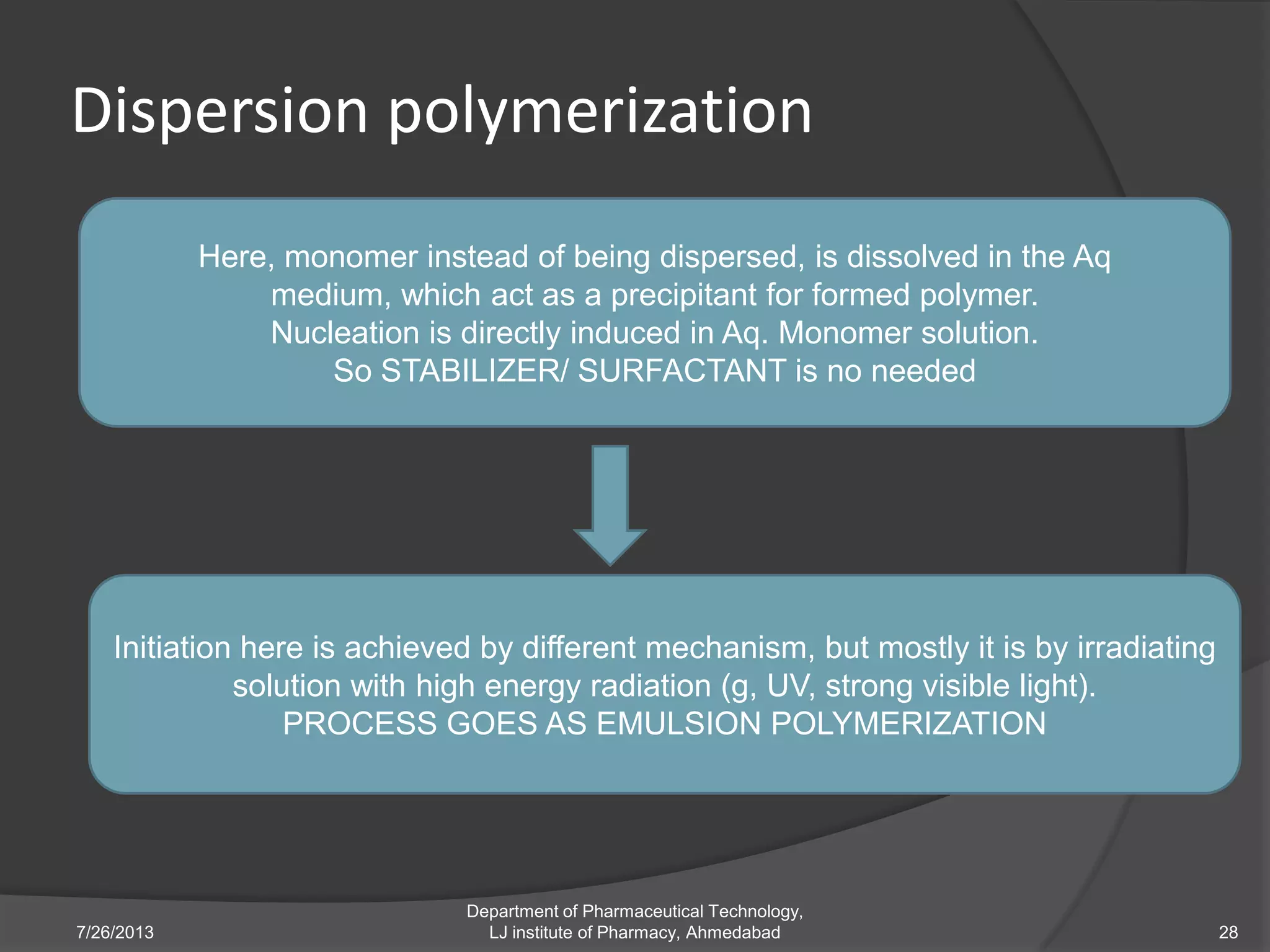
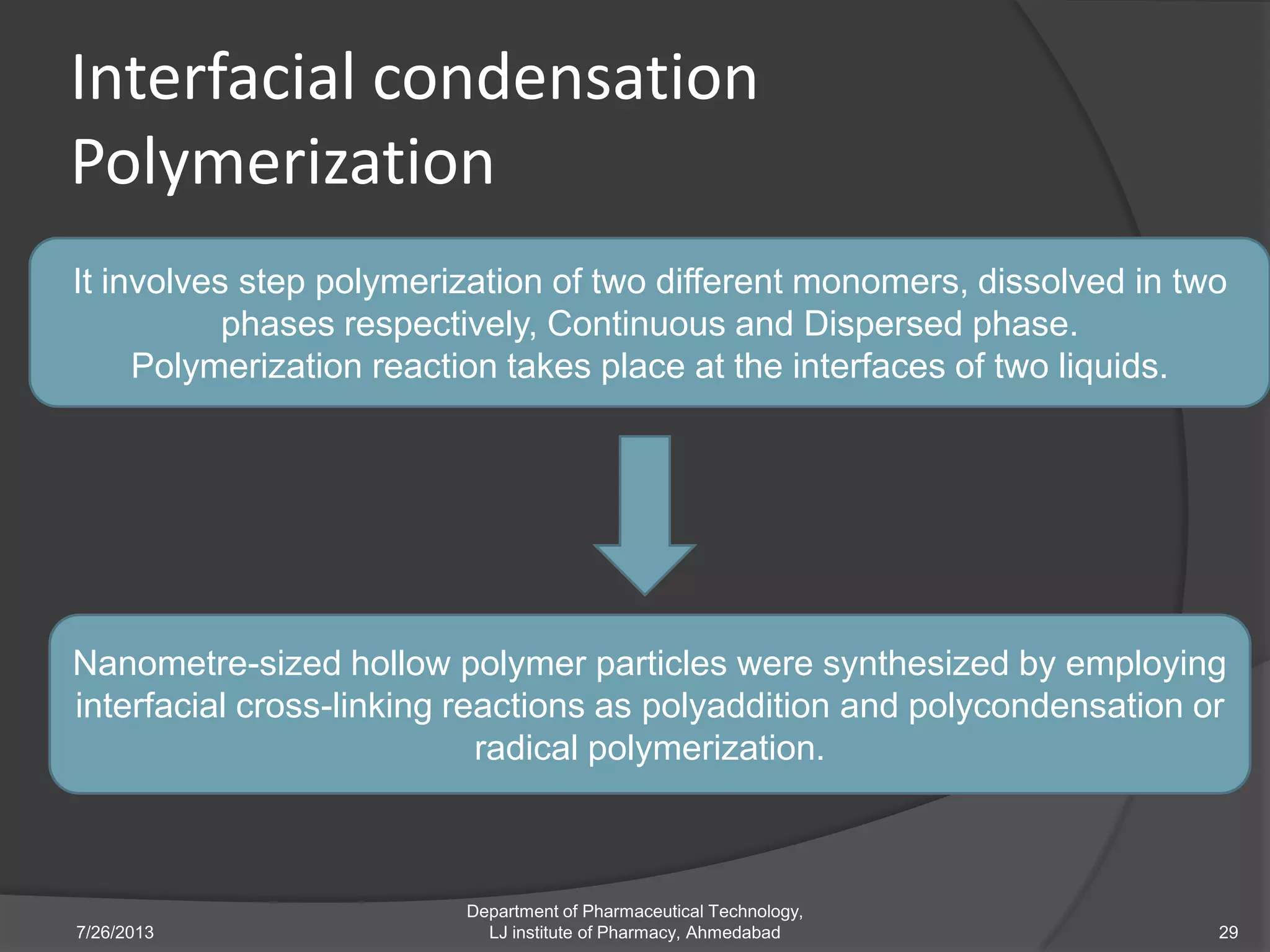
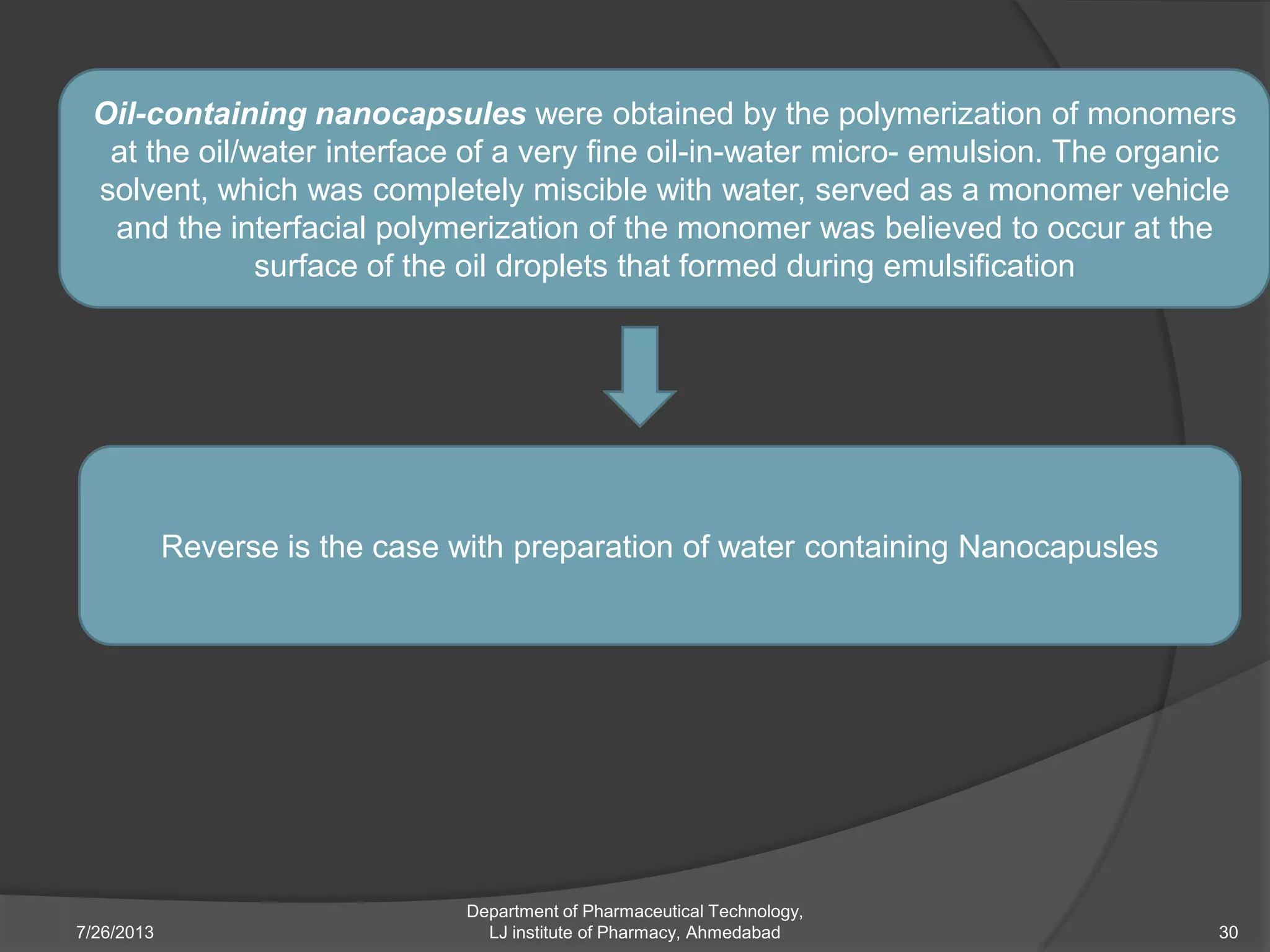
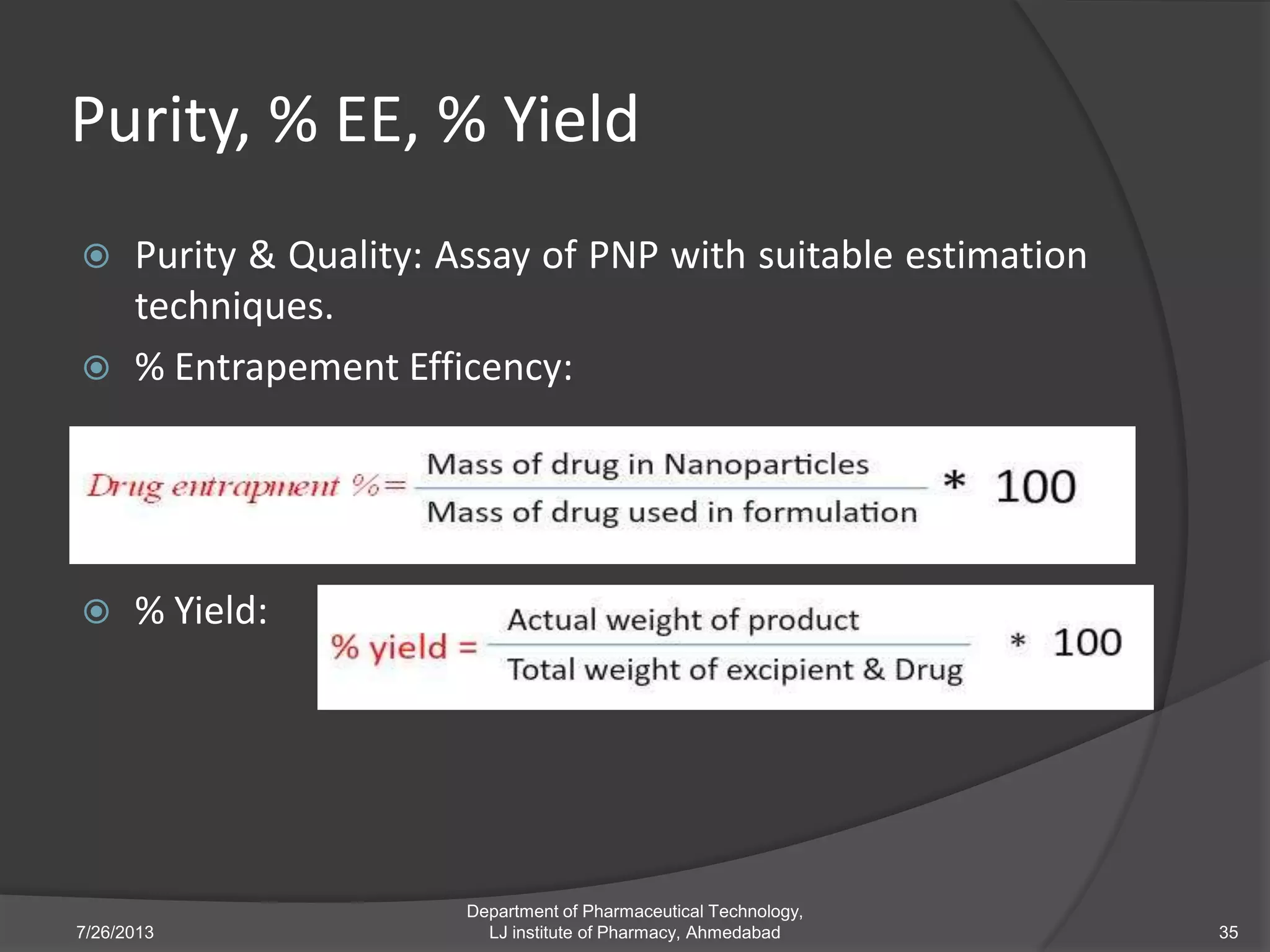
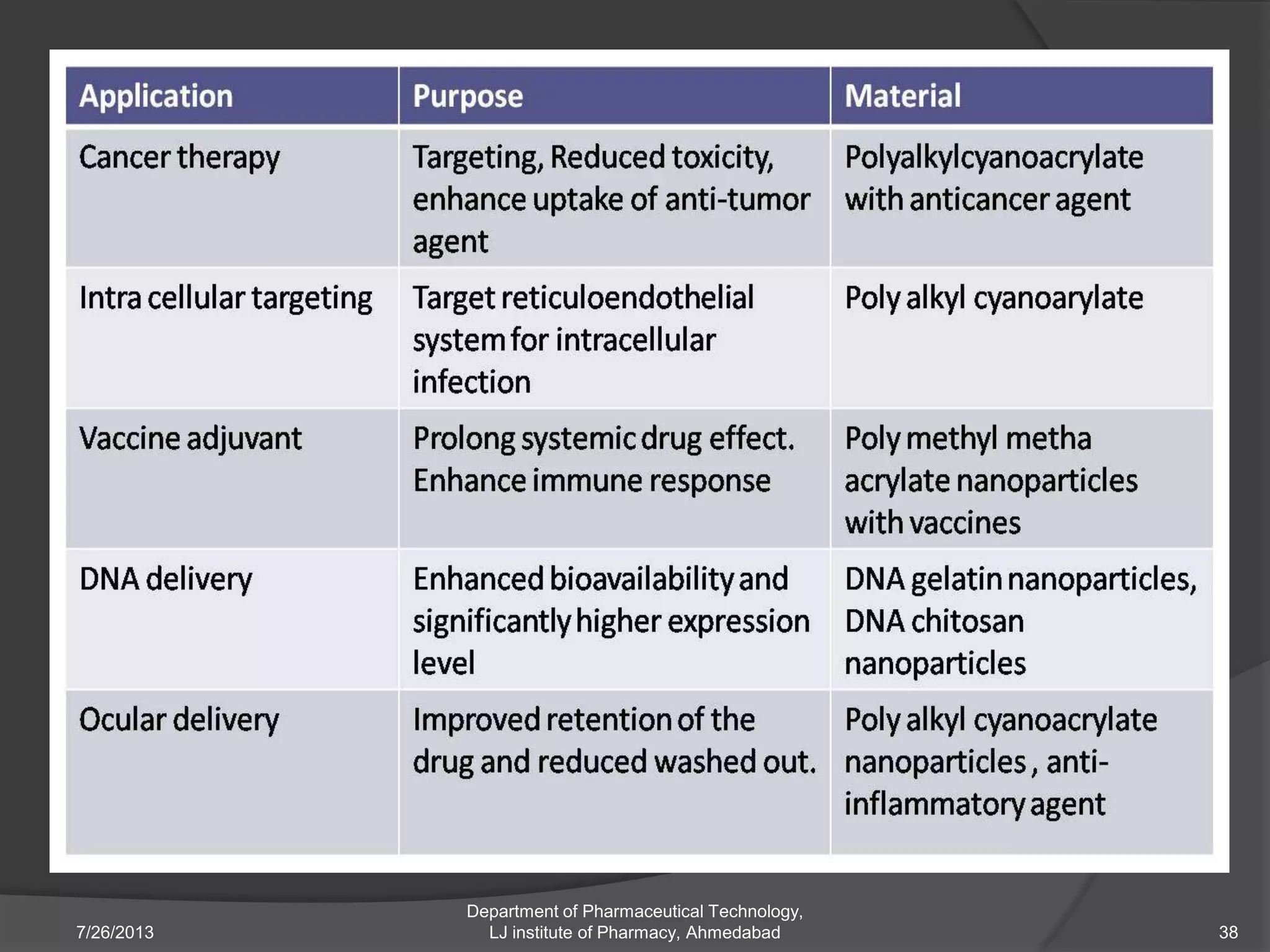
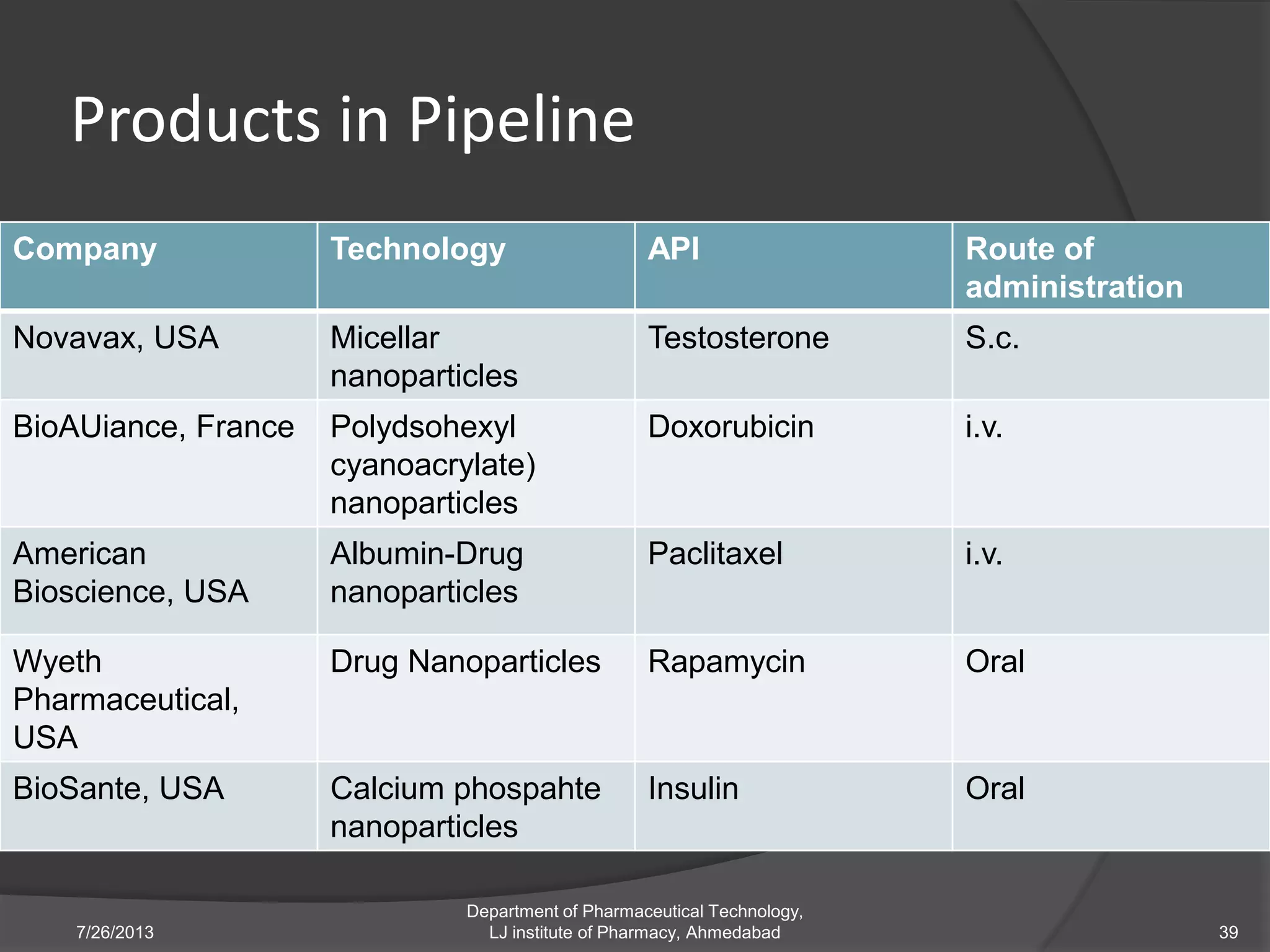
This document provides an overview of polymeric nanoparticles (PNPs). It defines PNPs and explains that drugs can be dissolved, entrapped, encapsulated, or attached to the nanoparticles. The advantages of PNPs for drug delivery are described, such as increased drug stability and targeting. Methods for preparing PNPs are outlined, including polymerization, precipitation, and cross-linking techniques. Characterization methods and applications of PNPs are also summarized briefly.