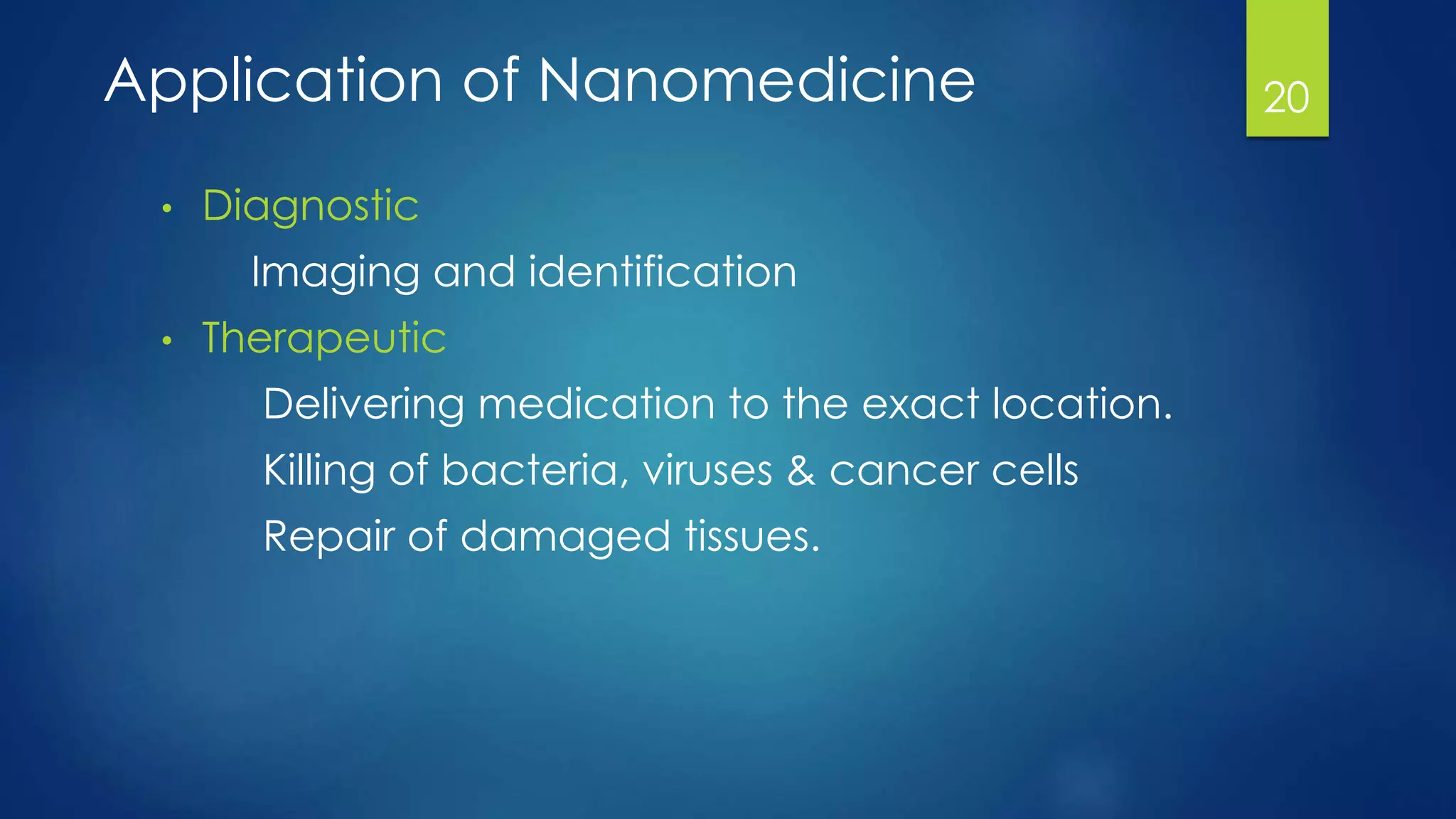
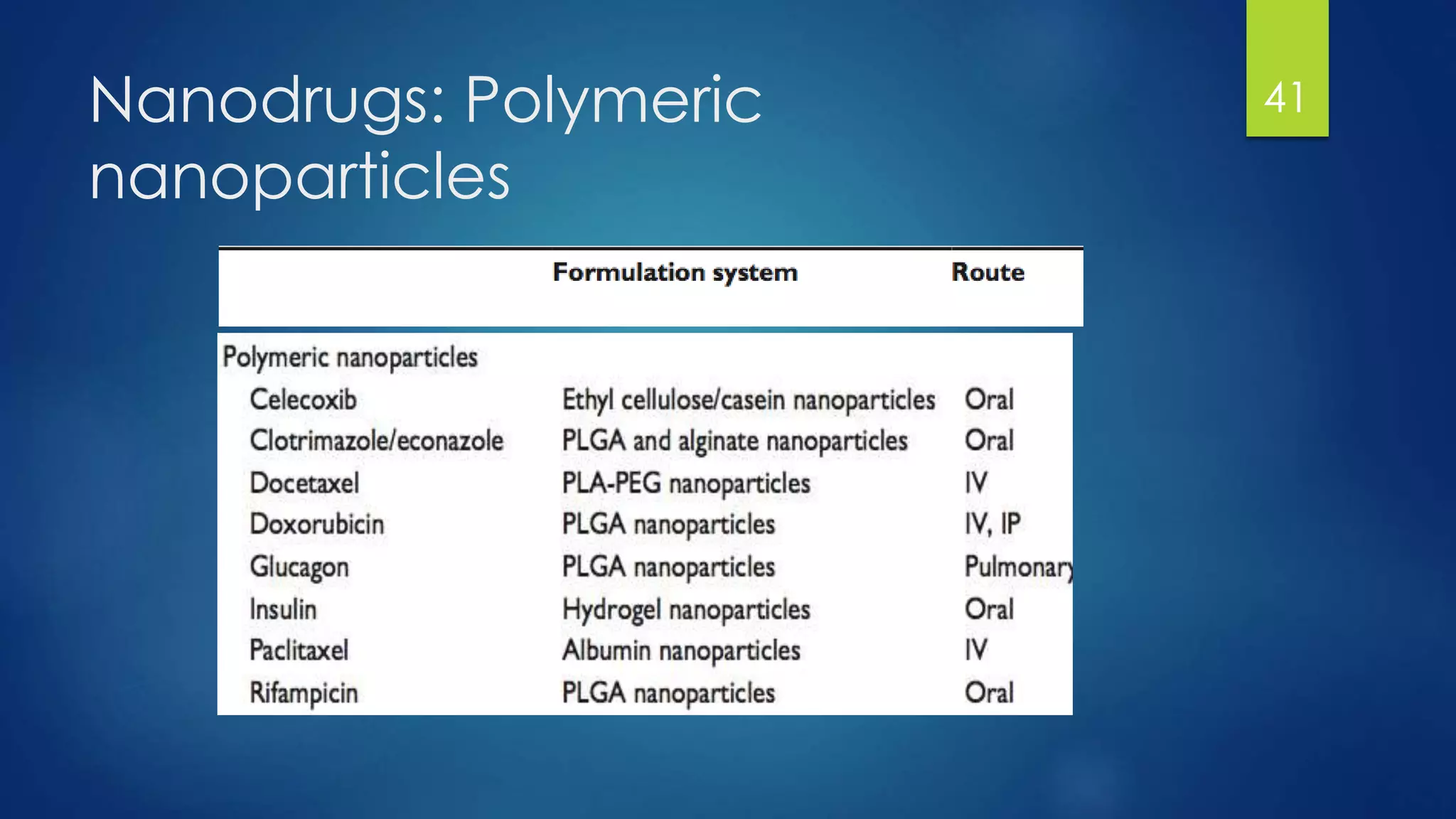
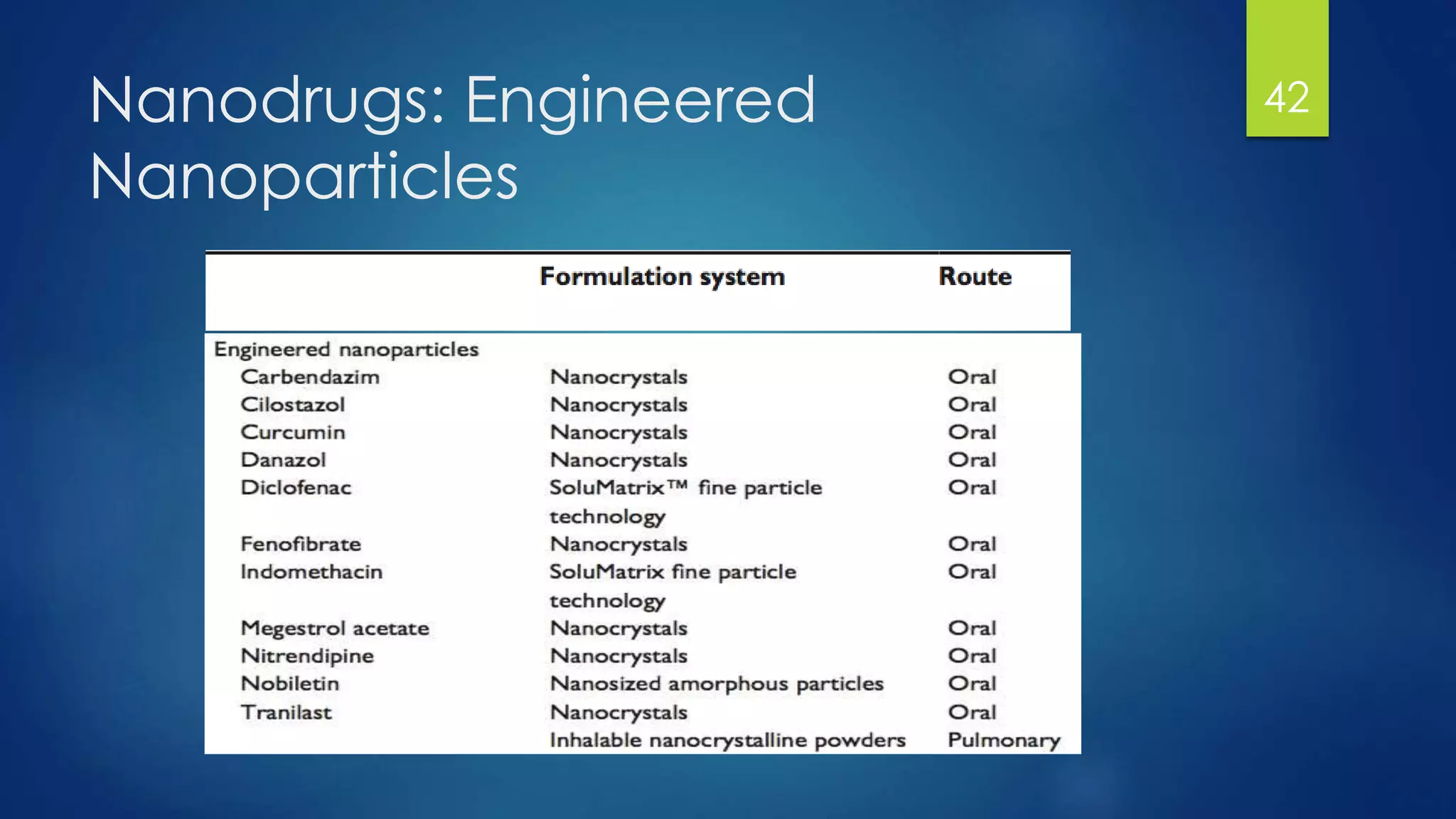
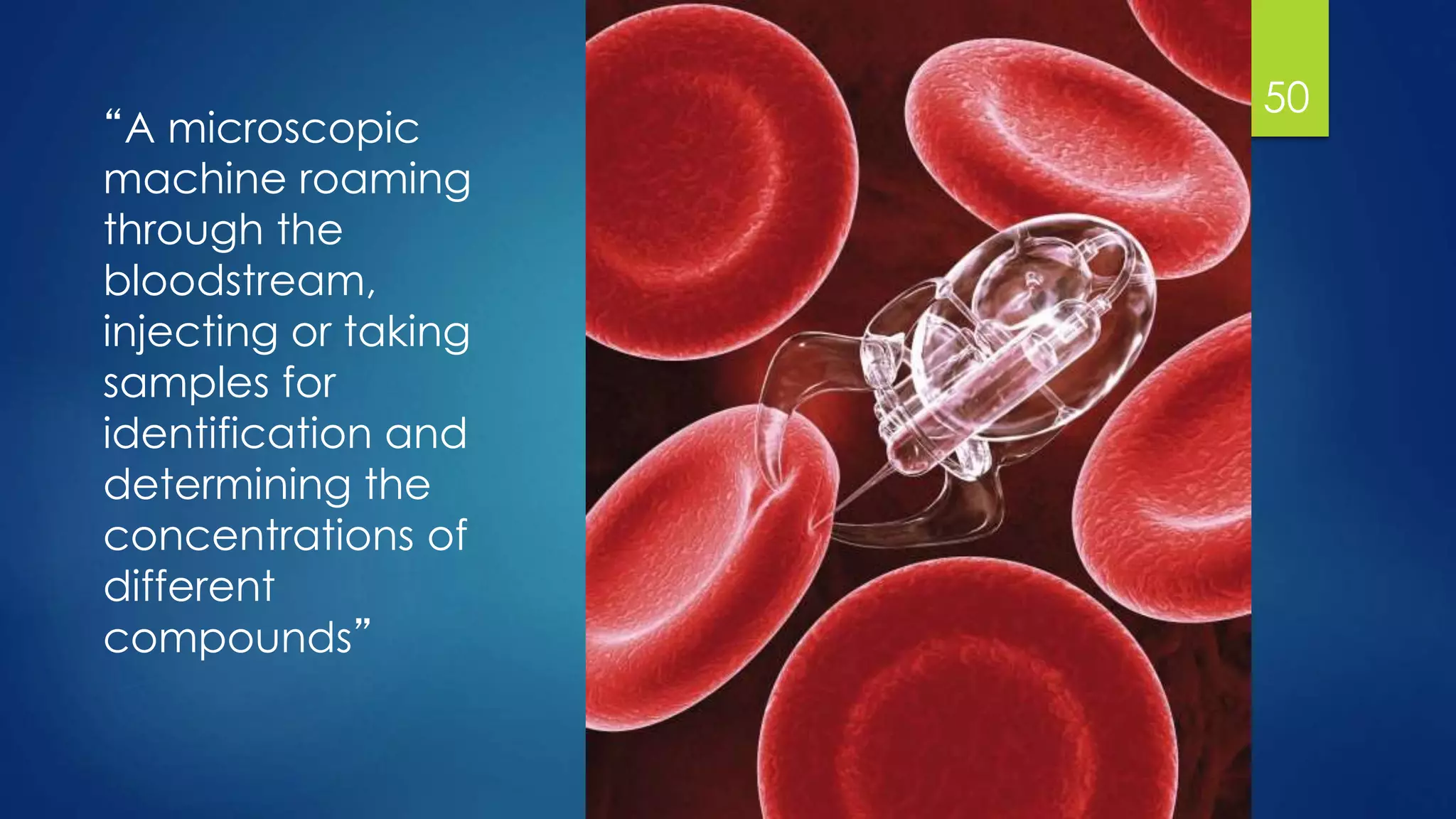
Nanomedicine is an interdisciplinary field that uses nanotechnology for medical applications. It aims to diagnose, treat, and prevent disease at the molecular level using nano-scale tools. The document outlines the history of nanomedicine from Richard Feynman's 1959 talk introducing nanotechnology to current applications. Key applications discussed include drug delivery using nanoparticles like liposomes, imaging contrast agents, and miniaturized medical devices. Challenges also remain around potential toxicities of nanomaterials.