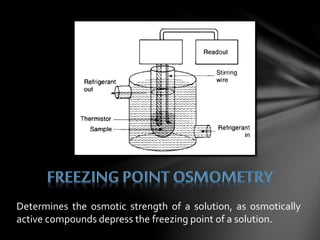
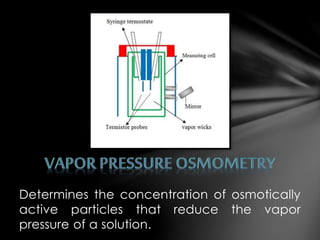
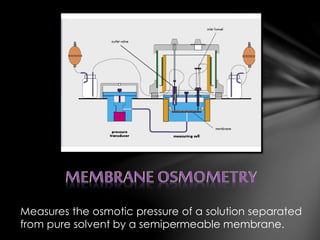
This document describes three laboratory techniques for determining the molecular weight of an unknown compound by measuring its osmotic strength in solution: osmometry, freezing point depression osmometry, and vapor pressure osmometry. Osmometry uses a semipermeable membrane to measure osmotic pressure, freezing point depression osmometry measures the degree to which a solution depresses the freezing point of a solvent, and vapor pressure osmometry detects differences in vapor pressure between a pure solvent and a solution. These techniques allow for determining the concentration of osmotically active particles in a solution.