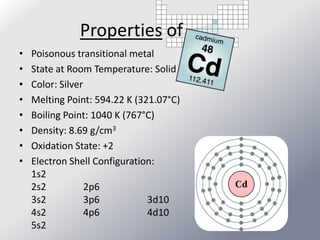
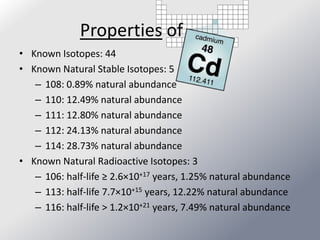
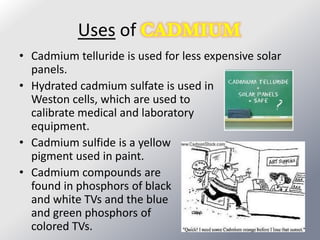
Friedrich Stromeyer discovered cadmium in 1817 while studying calamine. Cadmium is a soft, silvery-white metal that occurs naturally as a byproduct of zinc mining. It has many industrial uses, including in nickel-cadmium batteries, solar panels, and nuclear reactors. Cadmium is toxic to humans and environmental exposure can occur through cigarette smoke, coal burning, contaminated food/water, and landfill runoff.