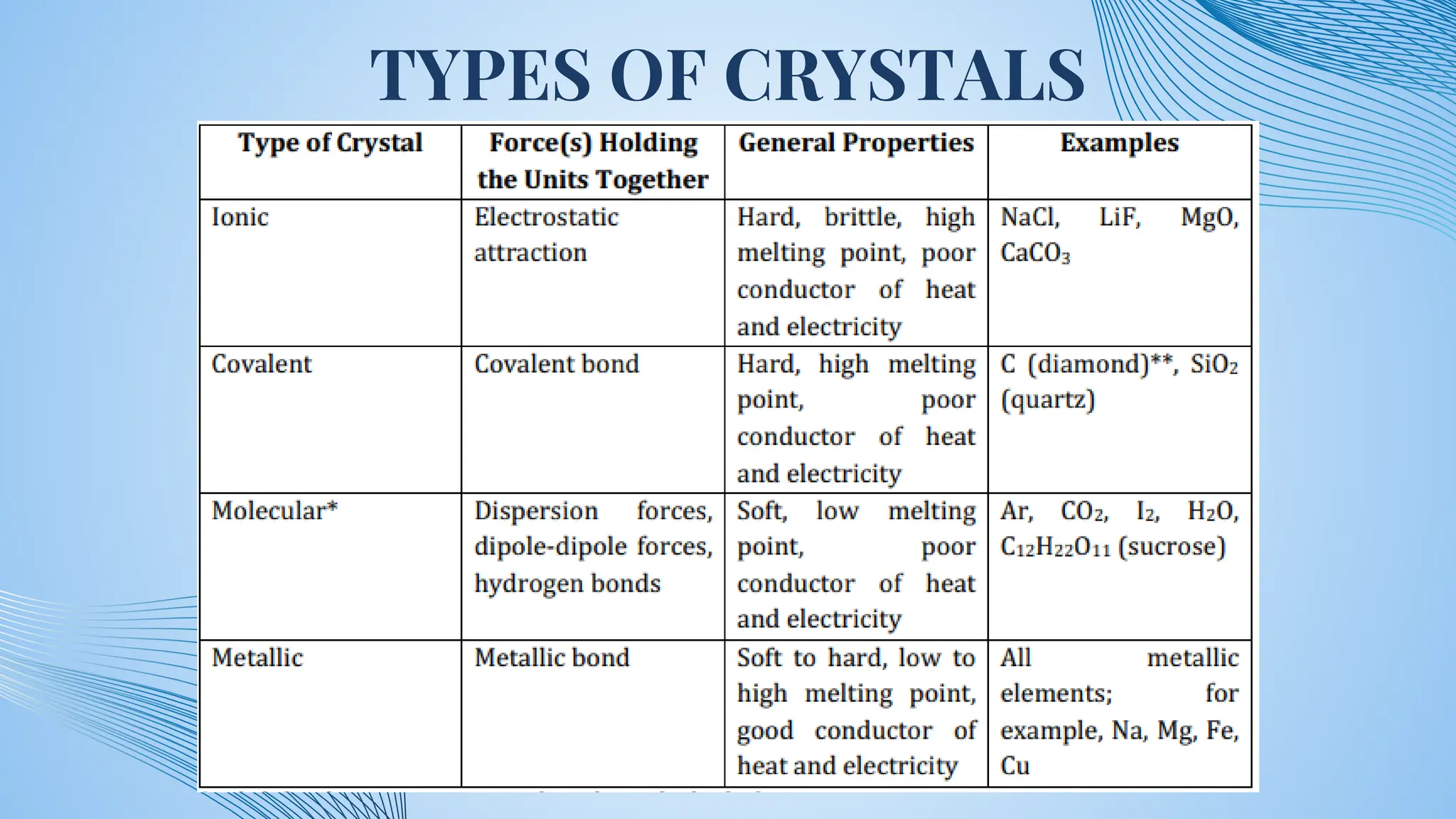
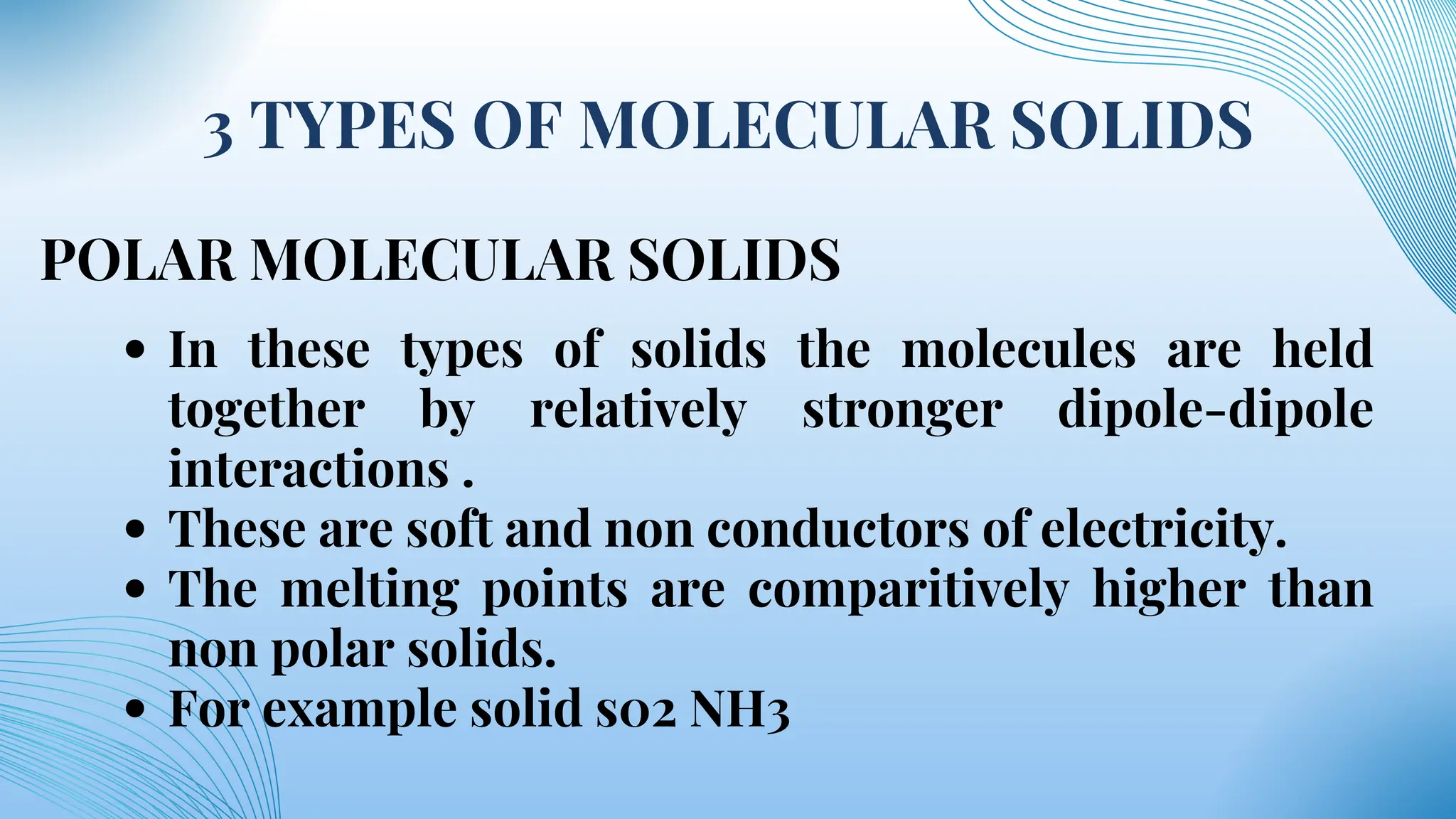
The document discusses various types of solids, specifically polar molecular solids, crystalline and noncrystalline materials, and metals, highlighting their properties, uses, and classification. It also covers toxic metals like cadmium, lead, mercury, iron, and zinc, detailing their occurrences, uses, and mechanisms of toxicity. Overall, the document emphasizes the structure, behavior, and applications of different solid-state materials and their environmental impacts.