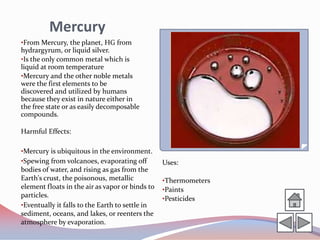
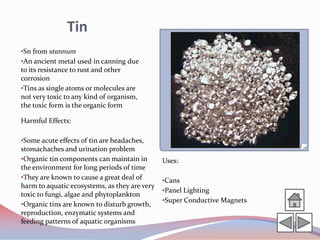
This document summarizes information about 10 different chemical elements: osmium, rhodium, copper, cadmium, mercury, lead, arsenic, fluorine, bromine, and tin. For each element, it provides details on the origin of the name, key chemical and physical properties, harmful health effects of exposure, and some common uses. The elements discussed range from metals to nonmetals and include several that are toxic or hazardous in certain forms.