Embed presentation
Downloaded 13 times



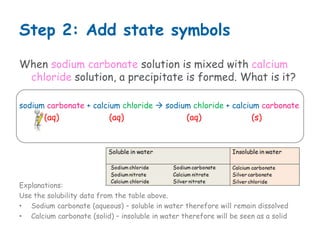
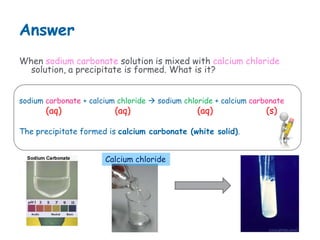
When sodium carbonate solution and calcium chloride solution are mixed, a precipitation reaction occurs where calcium carbonate forms as an insoluble solid precipitate. The chemical equation is: sodium carbonate + calcium chloride → sodium chloride + calcium carbonate (s). Calcium carbonate is the precipitate that forms.




