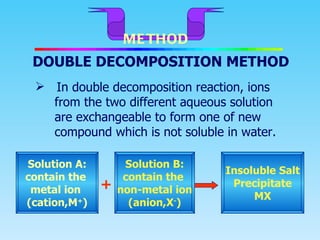
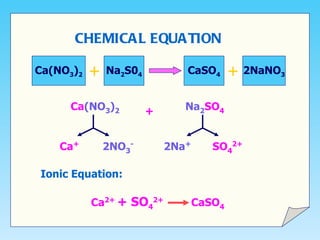
This document describes the double decomposition method for forming insoluble salts. It involves mixing two aqueous solutions containing different metal ions that exchange to form an insoluble salt precipitate. Specifically, it provides the example of mixing lead (II) nitrate and potassium iodide solutions to form lead (I) iodide precipitate. The procedure involves mixing the solutions, filtering out the precipitate, washing it with water, and drying to obtain pure salt crystals.