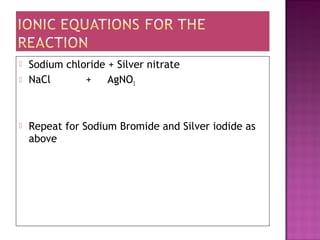
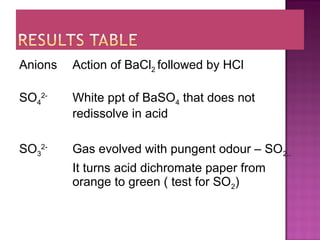
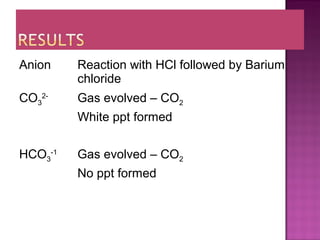
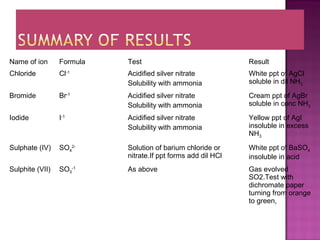
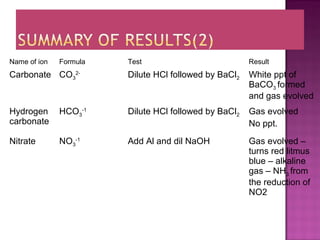
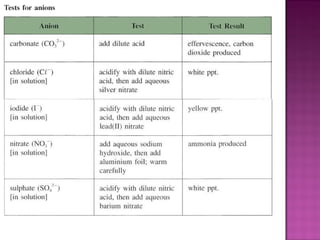
This document provides information about qualitative analysis of common anions and cations. It describes a series of chemical tests to identify various ions by observing reactions such as formation of precipitates or gases. For example, chloride ions are identified by the formation of a white precipitate with silver nitrate that dissolves in dilute ammonia. The document also lists the expected observations for ions such as sulfate, sulfite, carbonate, hydrogen carbonate and nitrate. Finally, it presents the analysis of an unknown salt and identifies it as chromium (III) carbonate based on the observed green precipitate and reactions.