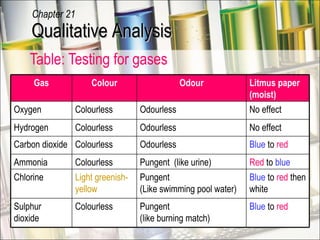
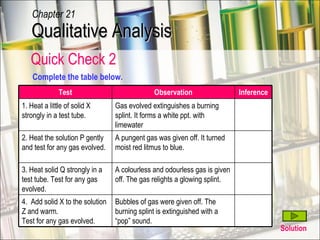
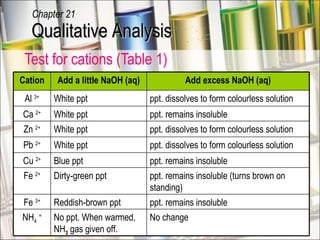
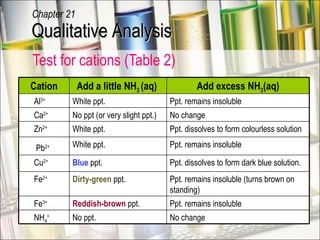
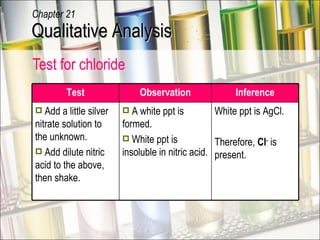
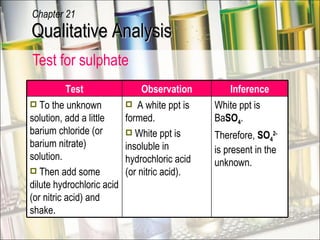
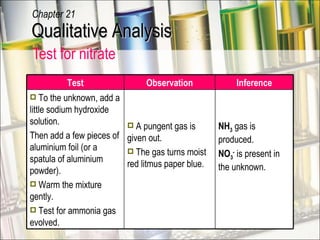
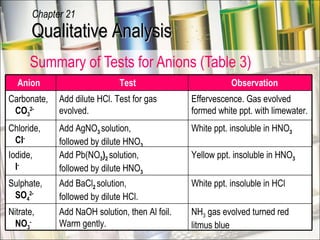
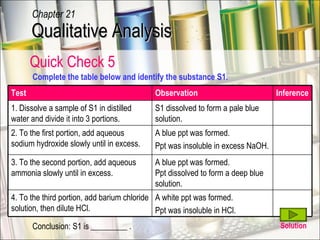
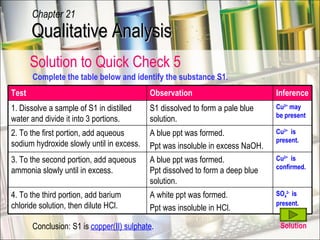
The document provides information about qualitative analysis (QA), which is a process chemists use to identify unknown substances. It describes the five main steps of QA: making preliminary observations, carrying out tests in a given order, recording observations, drawing conclusions, and identifying the unknown. It also provides details on identifying common gases, cations, and anions through a series of chemical tests and observations of color changes, precipitate formation, gas evolution and more.