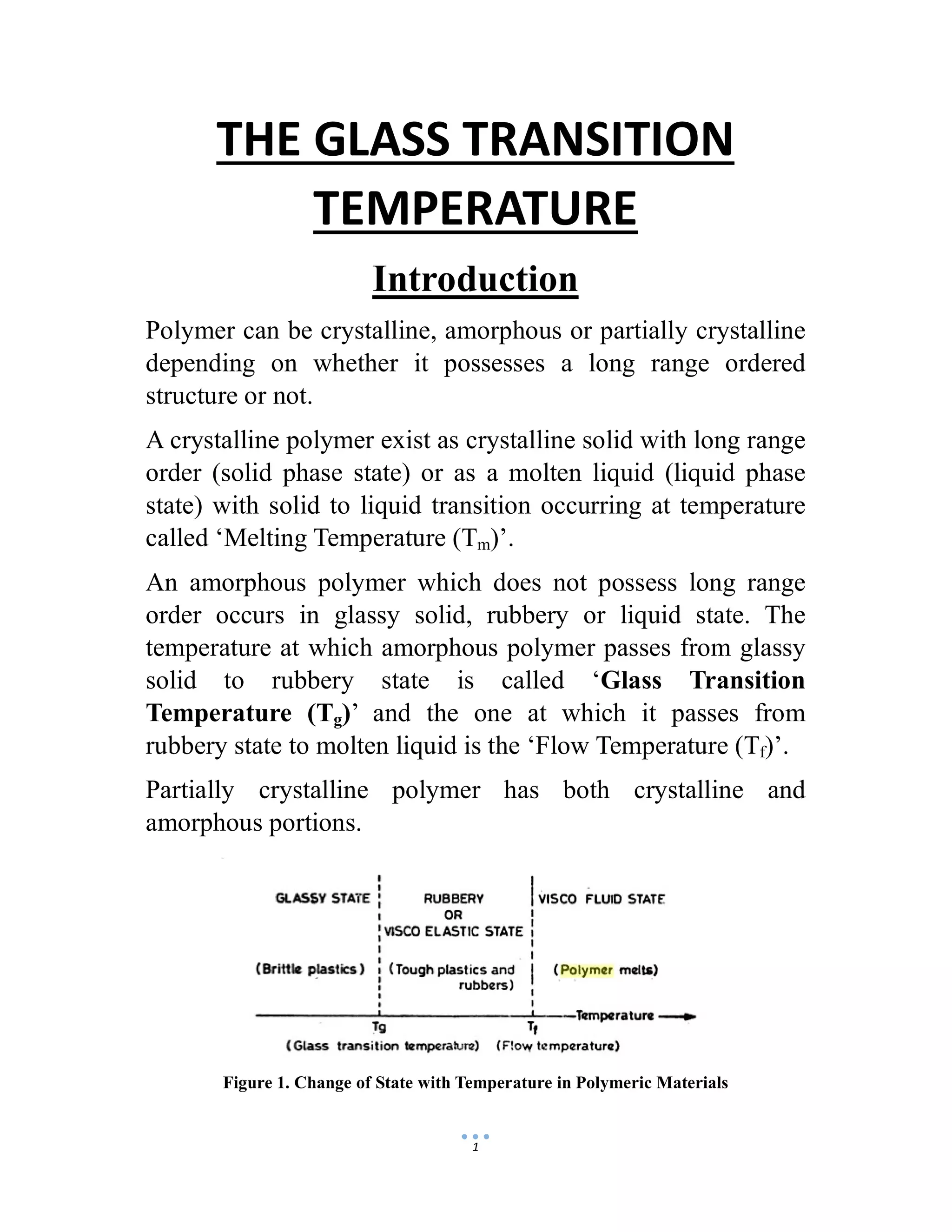
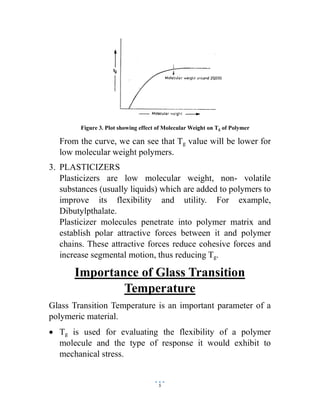
The document discusses the glass transition temperature (Tg) of polymers. Tg is the temperature at which an amorphous polymer transitions from a brittle, glassy state to a rubbery, flexible state. It depends on factors like the polymer's chemical structure, molecular weight, and presence of plasticizers. Knowing the Tg is important as it indicates the physical state of the polymer and suitable processing conditions. It also provides information about the polymer's flexibility and how it will respond to mechanical stresses.