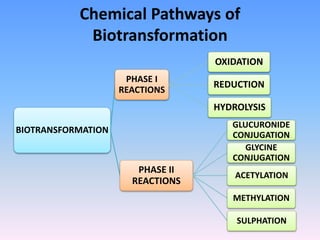
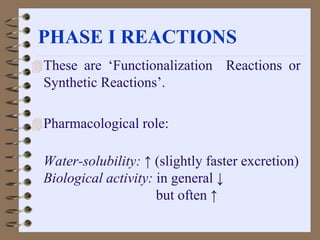
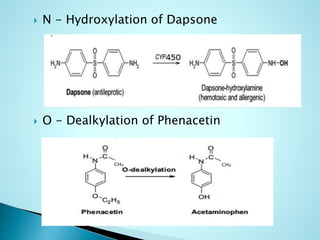
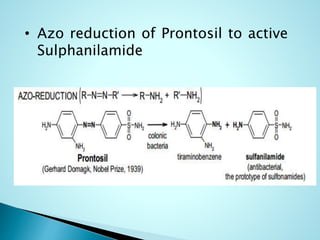
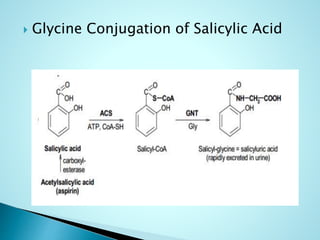
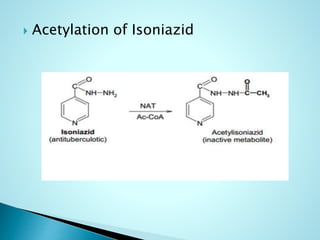
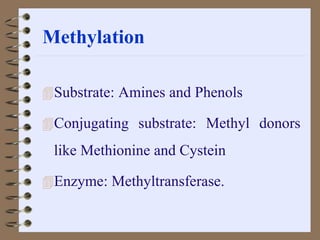
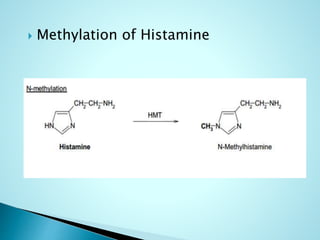
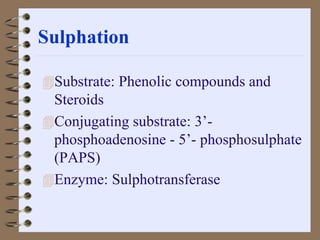
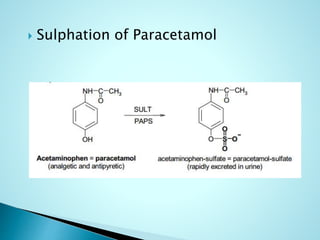
This document summarizes a seminar on biotransformation. It introduces biotransformation as the process by which substances are transformed chemically within the body. It outlines the two phases of biotransformation - Phase I involves functionalization reactions like oxidation, reduction and hydrolysis. Phase II involves conjugation reactions like glucuronide conjugation, glycine conjugation, acetylation and methylation that increase water solubility. The importance of biotransformation is that it can activate prodrugs, deactivate toxins, or sometimes produce toxic metabolites, and can also help break down pollutants in the environment.