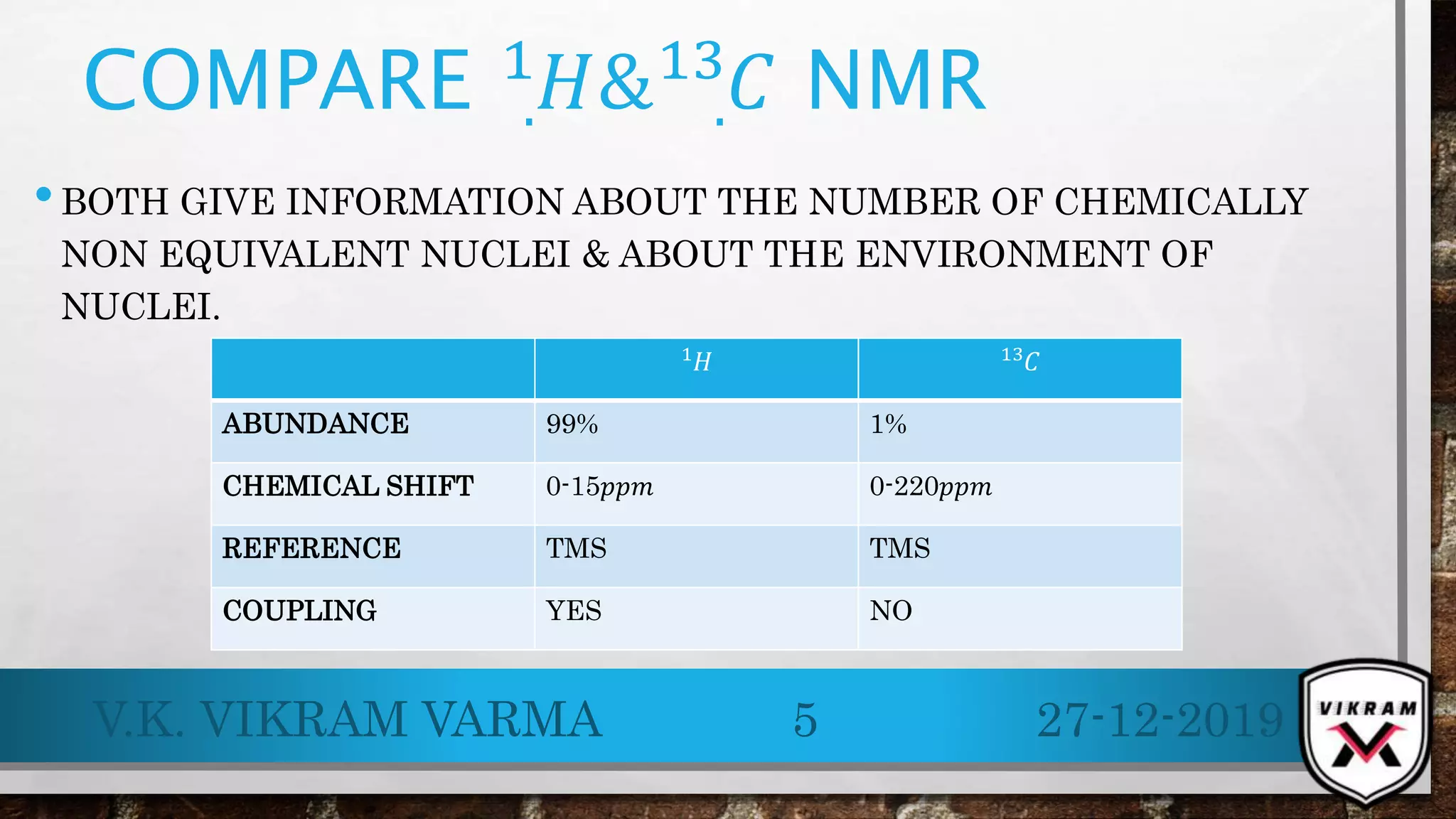
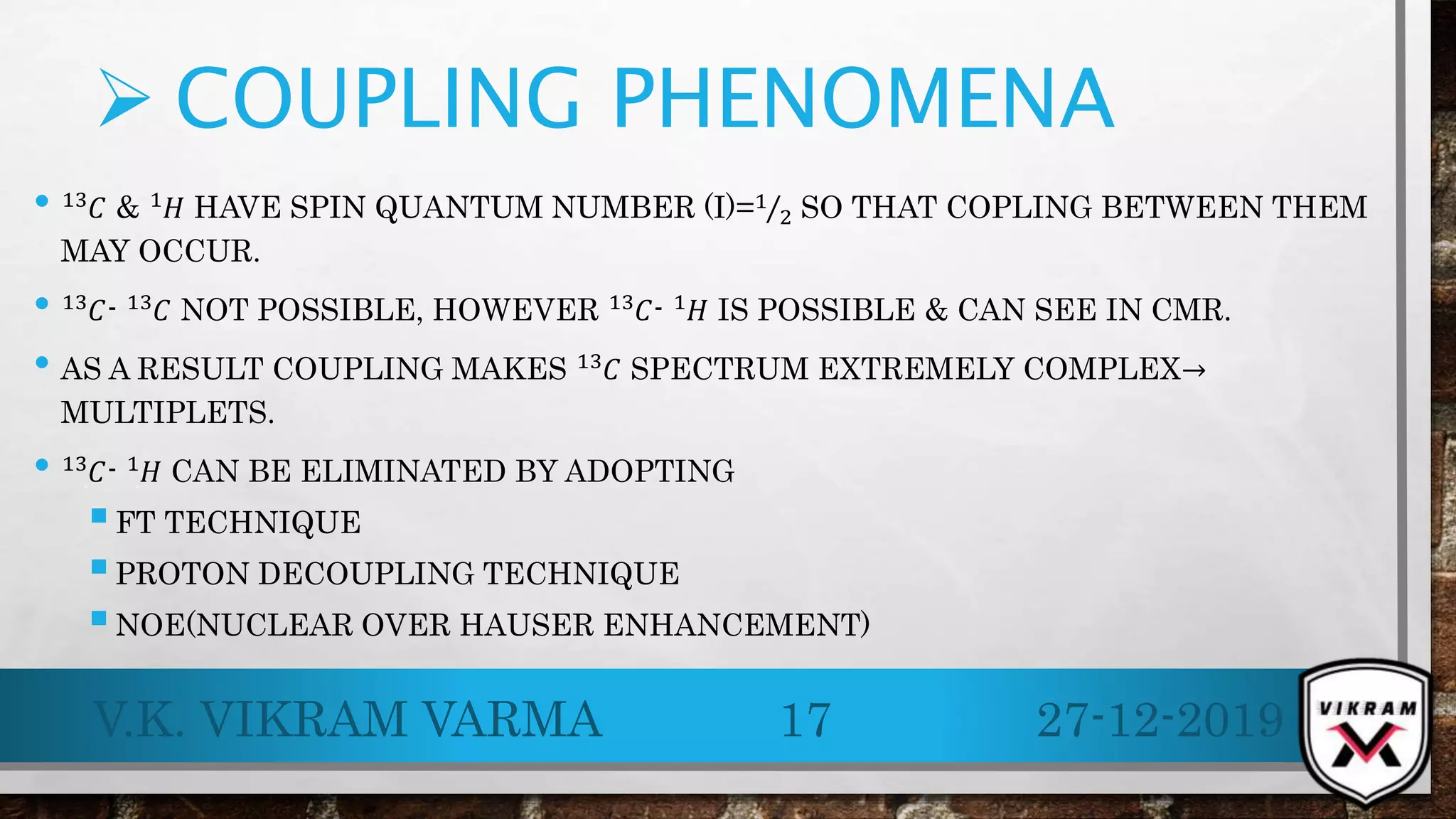
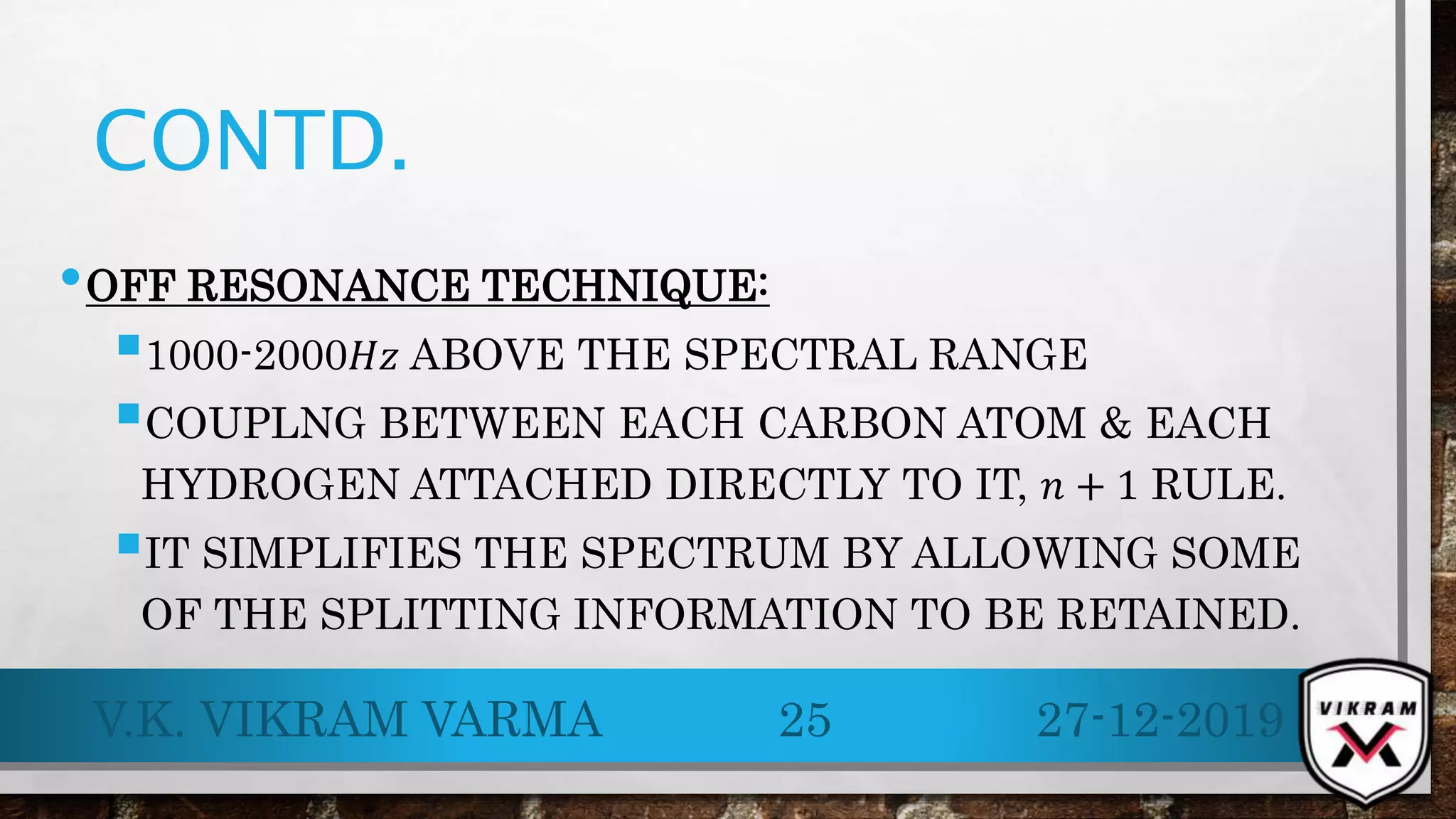
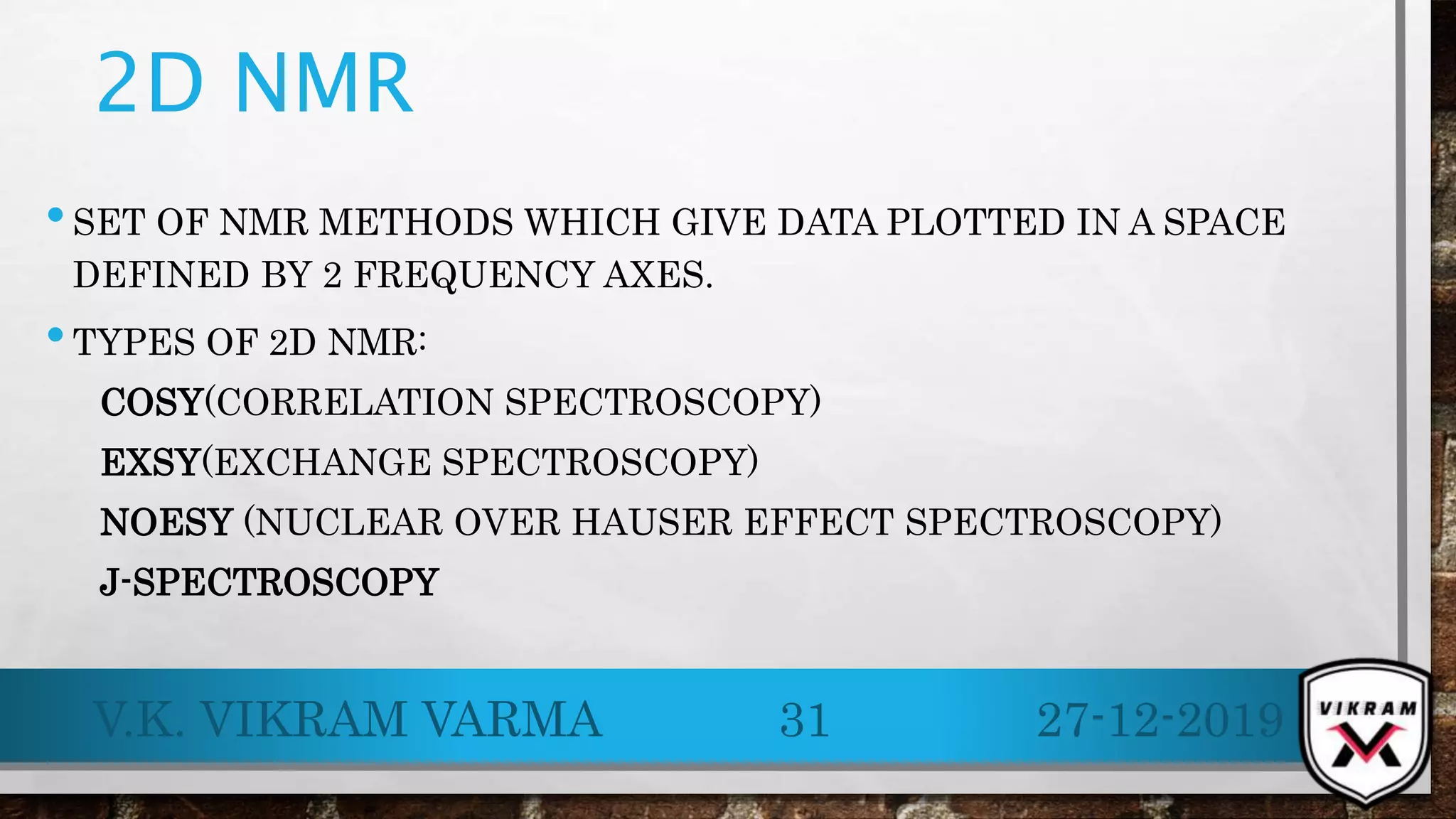
.13C NMR provides essential insights into carbon skeletons with signals spanning a range of 0-220 ppm, despite its lower sensitivity compared to .1H NMR. Challenges like natural abundance and coupling phenomena can complicate .13C NMR, but techniques such as Fourier Transform and decoupling can enhance resolution and clarity of spectra. Its advantages over .1H NMR make it valuable for analyzing complex molecular structures and conducting various applications, including metabolic studies.