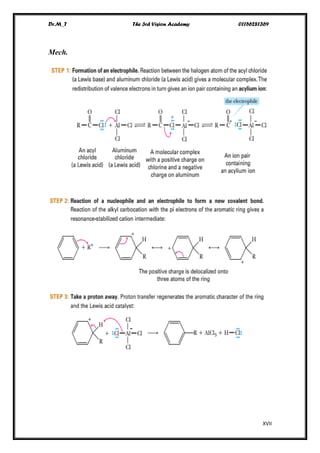
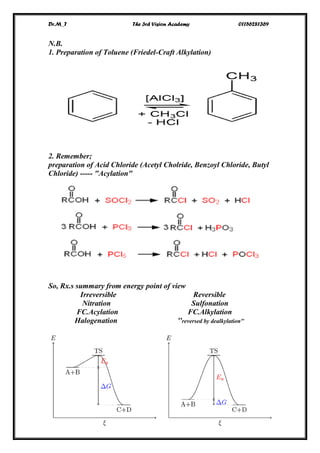
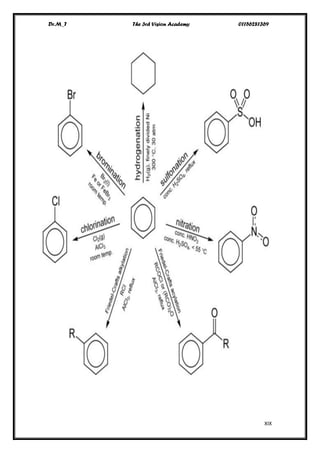
The document discusses aromatic compounds, focusing on annulenes and the application of Hückel's rule to assess their aromaticity. It covers characteristics of various aromatic ions, methods of electrophilic substitution reactions like bromination, nitration, and sulfonation, as well as the Friedel-Crafts reactions for alkylation and acylation. Additionally, it addresses the structural aspects of fullerenes and nanotubes, highlighting their unique properties and applications.
![Dr.M_T The 3rd Vision Academy 01156281369
I
Aromatic Compound
Classes of Aromatic comp.,,,
I. Annulens
-Def;
"completely conjugated monocyclic polyenes."
"cyclic hydrocarbon with continuous alternating of single and double
bond."
Cyclobutadiene, benzene, and cyclooctatetraene are the first members
of a family of annulenes.
-Nomenclature;
[ no. of C-atoms ] + annulene
-Note;
*Aromaticity in the larger annulenes depends on whether the molecule
can adopt the necessary planar conformation.
# carbon atoms must be EVEN (4,6,8 …) to be AROMATIC.
*Geometry of the structure control hybridization concept not vice vesra.
*Polyenes;
" poly-unsaturated organic compounds that contain at least three al-
ternating double and single carbon–carbon bonds, and have conjugated
system."
*The generality and limits of the Hückel rule can be tested by consider-
ing the properties of the annulene series.
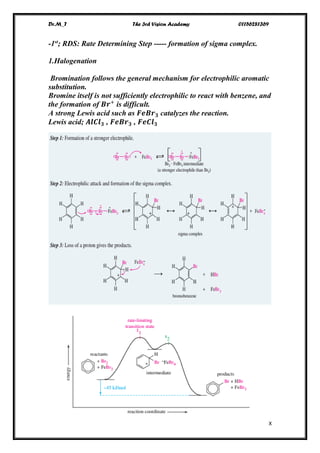
e.g.
cyclobutadiene Benzene cyclooctatetraiene
[4]annulene [ 6]annulene [ 8]annulene
n=0.5 n=1 n=1.5
antiaromatic aromatic antiaromatic](https://image.slidesharecdn.com/lec-170104121848/75/Aromatic-Comp-Lec-2-1-2048.jpg)
![Dr.M_T The 3rd Vision Academy 01156281369
II
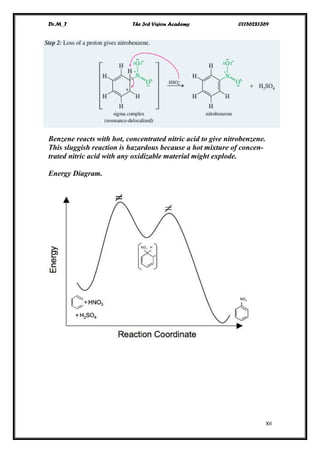
[10]annulene [12]annulene [14]annulene
n=2 n=2.5 n=3
aromatic antiaromatic aromatic
[16]annulene [18]annulene
n=3.5 n=4
antiaromatic aromatic
N.B.
-The [10]annulene isomer with two trans double bonds cannot adopt a
planar conformation either, because two hydrogen atoms interfere with
each other. Neither of these [10]annulene isomers is aromatic, even
though each has pi electrons, with If the interfering hydrogen atoms in
the partially trans isomer are removed, the molecule can be planar.
[10]annulene [10]annulene [10]annulene
all CIS two trans remved interfering H
n=2 n=2 n=2
nonaromatic nonaromatic aromatic
-Naphthalene is the result when the interfering
hydrogen atoms of [10]annulene are replaced
with a bond.](https://image.slidesharecdn.com/lec-170104121848/85/Aromatic-Comp-Lec-2-2-320.jpg)
![Dr.M_T The 3rd Vision Academy 01156281369
III
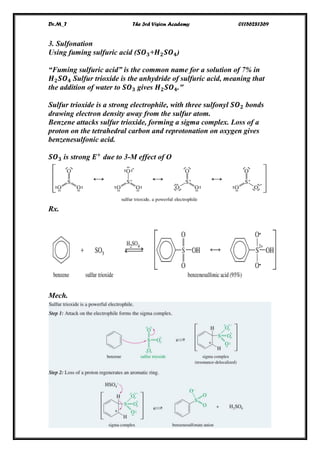
-[18]Annulene offers a particularly significant test of
the Hückel rule. The internal cavity in [18]annulene is
large enough to minimize steric interaction between
the internal hydrogens in a geometry that is free of
angle strain.
[18]annulene
-Hückel’s rule also applies to systems having odd numbers of carbon
atoms and bearing positive or negative charges
II. Aromatic Ions
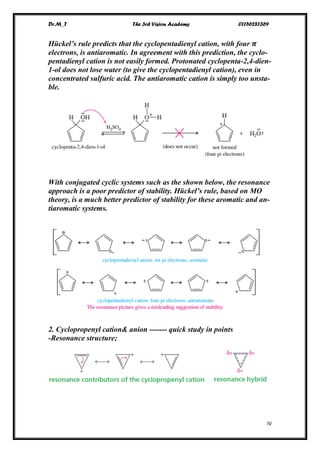
1. The Cyclopentadienyl Ions
With four 𝝅electrons (a cation), Hückel’s rule predicts this system to be
antiaromatic.
With six 𝝅electrons (an anion), Hückel’s rule predicts aromaticity.
Because the cyclopentadienyl anion (six 𝝅electrons) is aromatic, it is
unusually stable compared with other carbanions. It can be formed by
abstracting a proton from cyclopentadiene, which is unusually acidic
for an alkene.](https://image.slidesharecdn.com/lec-170104121848/85/Aromatic-Comp-Lec-2-3-320.jpg)