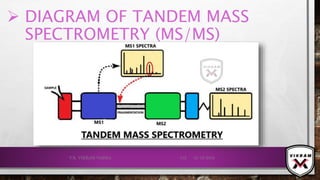
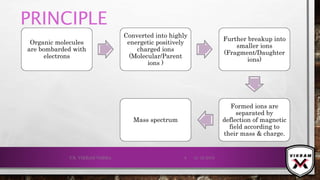
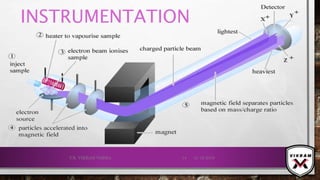
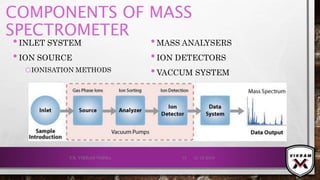
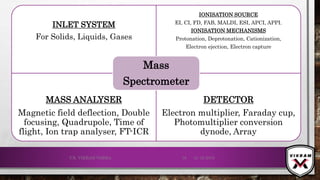
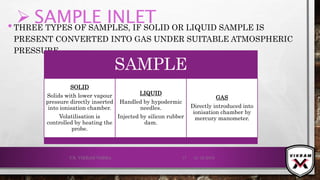
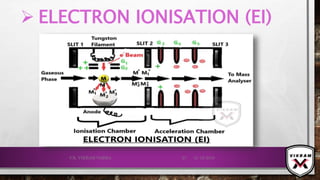
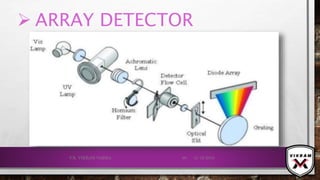
The document provides an overview of mass spectrometry. It discusses the history, principles, instrumentation, ionization techniques, mass analyzers, and applications of mass spectrometry. Mass spectrometry involves converting sample molecules to ions, separating the ions based on their mass-to-charge ratio, and detecting the ions. Key components include an ion source, mass analyzer, and ion detector. Common ionization methods include electron ionization and chemical ionization. Common mass analyzers are magnetic sector, quadrupole, time-of-flight, and ion trap. Mass spectrometry has various applications in fields like proteomics, metabolomics, and environmental analysis.







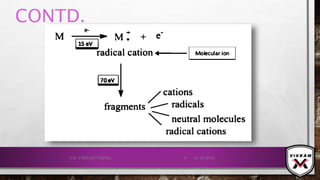
![CONTD.
Neopentane
(C5H12)
CH
+
3
+C4H9⦁
C2H
+
5
+
C3H7⦁
C3H
+
7
+
C2H5⦁
C4H
+
9
+ CH
⦁
3
[C5H12]⦁+
21-12-2019V.K. VIKRAM VARMA 10
Fragmentation
example
Electron bombardment
⦁ Cations
⦁ Free radicals](https://image.slidesharecdn.com/massspectrometry-191221153343/85/Mass-spectrometry-Analytical-Technique-10-320.jpg)
















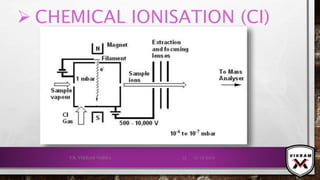





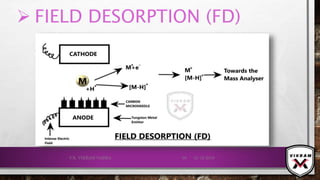

![CONTD.
ION FORMATION TAKES PLACE MAINLY BY 2 MECHANISMS:
FIELD IONISATION: ELECTRONS ARE REMOVED FROM THE SPECIES/
ANALYTE IN A HIGH ELECTRIC FIELD.
𝑴 → 𝑴+ + ⅇ−
CATIONS ATTACHED: CATIONS WILL BE ATTACHED WITH ANALYTE
MOLECULE 𝑖. 𝑒. 𝐻+
𝑜𝑟 𝑁𝑎+
𝑒𝑡𝑐.
𝑴 + 𝑯+
→ [𝑴 − 𝑯]+
POSITIVE IONS WILL BE REPELLED BY THE ANODE & THEY WILL
GO TOWARDS THE MASS ANALYSER.
IONS DOESN’T HAVE SUFFICIENT INTERNAL ENERGY FOR
FRAGMENTATION, DUE TO THIS STABLE IONS FORMED.
21-12-2019V.K. VIKRAM VARMA 40](https://image.slidesharecdn.com/massspectrometry-191221153343/85/Mass-spectrometry-Analytical-Technique-40-320.jpg)

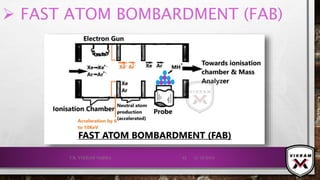

![CONTD.
•METHODOLOGY:
CHARACTERISTICS OF THE MATRIX:
NON VOLATILE
LOW VAPOUR PRESSURE LIQUID
EXAMPLES: GLYCEROL, THIOGLYCEROL, DIMETHANOLAMINE, TRIETHANOLAMINE.
𝑋𝑒 OR 𝐴𝑟 (ACCELERATED NEUTRAL ATOMS)WILL BE BOMBARDED TO THE SAMPLE
MATRIX MIXTURE & IONISE THE SAMPLE DUE TO TRANSLATIONAL ENERGY. E.G.:
𝑿ⅇ + ⅇ− → 𝑿ⅇ⦁ + 𝟐ⅇ−
𝑿ⅇ + 𝑿ⅇ+⦁ → 𝑿ⅇ + 𝑿ⅇ+⦁
𝑮𝒍𝒚𝒄ⅇ𝒓𝒐𝒍 − 𝑯+
→ [𝑴𝑯]+
•IF 𝐶𝑠+
ION IS USED THAN IT IS KNOWN AS SIMS (SECONDARY
IONISATION MASS SPECTROMETRY).
21-12-2019V.K. VIKRAM VARMA 44](https://image.slidesharecdn.com/massspectrometry-191221153343/85/Mass-spectrometry-Analytical-Technique-44-320.jpg)




![CONTD.
• MIXED WITH POLAR MATRIX & LASER LIGHT IS USED FOR IONISATION.
• SOFT IONISATION METHOD, WHICH USES PULSED LASER BEAM.
• DETERMINE THE MOLECULAR WEIGHT OF PEPTIDES, ANTIBODIES,
PROTEIN, MOLECULES 𝑒𝑡𝑐 UP TO THE SIZE OF 300𝐾𝐷𝑎.
• LASER BEAM WILL HIT THE SAMPLE: MATRIX MIXTURE & ANALYTE/
SAMPLE WILL CONVERT INTO THE FORM OF GAS.
• ANALYTE/ SAMPLE & MATRIX WILL ALSO CONVERTS INTO THE IONS
DUE TO TRANSITIONAL ENERGY.
PROTONATION: 𝑴 + 𝑯+ → [𝑴𝑯]+
DEPROTONATION: 𝑴 → [𝑴 − 𝑯]−+𝑯+
21-12-2019V.K. VIKRAM VARMA 49](https://image.slidesharecdn.com/massspectrometry-191221153343/85/Mass-spectrometry-Analytical-Technique-49-320.jpg)











![CONTD.
CORONA DISCHARGE ELECTRODE 𝒐𝒓 𝜷 −PARTICLE EMITTER IS
USED FOR IONISATION.
DUE TO COLLISION & ION MOLECULAR CHARGE TRANSFER
BETWEEN SOLVENT & ANALYTE TAKES PLACE & IT WILL
PRODUCE
𝑴𝑯+
𝒊𝒐𝒏 𝑨 + 𝑺+
→ 𝑴𝑯+
+ 𝑺−
𝒑𝒐𝒔𝒊𝒕𝒊𝒗ⅇ
[𝑴 − 𝑯]− 𝒊𝒐𝒏 𝑨+ + 𝑺 → [𝑴 − 𝑯]−+𝑺𝑯+ 𝒏ⅇ𝒈𝒊𝒕𝒊𝒗ⅇ
•APCI IS USED TO ANALYSE POLAR, THERMOSTABLE SUBSTANCE
WITH MOLECULAR WEIGHT LESS THAN 1500DALTONS.
21-12-2019V.K. VIKRAM VARMA 62](https://image.slidesharecdn.com/massspectrometry-191221153343/85/Mass-spectrometry-Analytical-Technique-62-320.jpg)
![CONTD.
ADVANTAGES
• MULTIPLE CHARGING IS TYPICALLY NOT
OBSERVED AS THE IONISATION PROCESS IS
MORE ENERGETIC THAN ESI.
• ELECTRON TRANSFER OR PROTON LOSS,
([𝑀 − 𝐻]−
) OCCURS IN THE NEGATIVE MODE.
• PROTON TRANSFER OCCURS IN THE POSITIVE
MODE.
• AT ATMOSPHERIC PRESSURE ANALYTE
MOLECULES COLLIDE WITH THE REAGENT
IONS FREQUENTLY & HENCE IONISATION IS
VERY DIFFICULT.
DISADVANTAGES
• RELATIVELY LOW ION CURRENTS.
• VERY SENSITIVE TO CONTAMINANTS
SUCH AS ALKALI METALS OR BASIC
COMPOUNDS.
• RELATIVELY COMPLEX HARDWARE
COMPARED TO OTHER ION SOURCES.
21-12-2019V.K. VIKRAM VARMA 63](https://image.slidesharecdn.com/massspectrometry-191221153343/85/Mass-spectrometry-Analytical-Technique-63-320.jpg)



















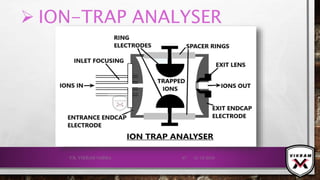




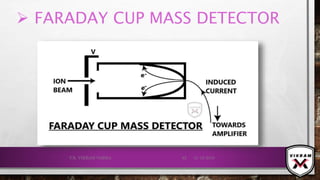

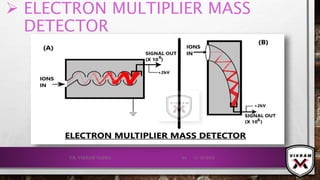

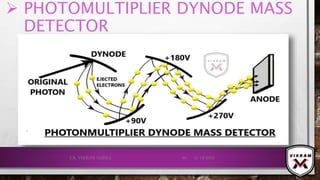






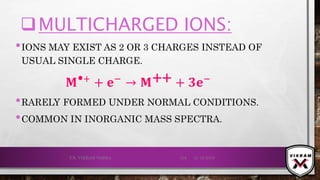
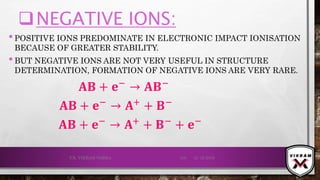
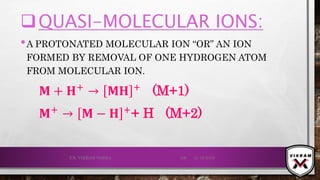
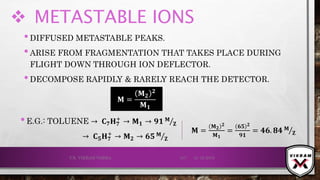



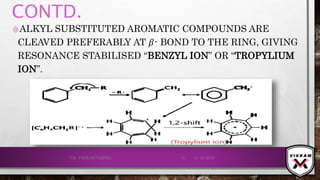
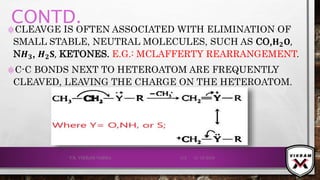

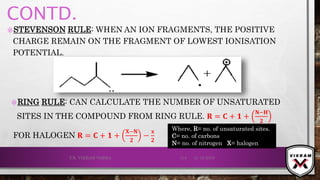









![ISOTOPIC PEAK•EACH ISOTOPE WILL SHOW UP AS A SEPARATE LINE IN MS.
•THE PRESENCE OF ISOTOPES READILY PRODUCE THE ISOTOPE
IONS IN THE SPECTRUM ACCOMPANIED BY A MAIN MOLECULAR
& FRAGMENT ION PEAK.
•.12
C – 98.9% NATURAL ABUNDANCE- VERY HIGH PEAK- M+
PEAK
(BASE PEAK).
•.13 C – 1.1% NATURAL ABUNDANCE- VERY LOW PEAK-
[M + 1]+PEAK.
•BASE PEAK IS THE LARGEST PEAK IN THE SPECTRUM &
INTENSITY OF EVERY OTHER PEAK IS REPORTED IN
COMPARISON TO BASE PEAK.
21-12-2019V.K. VIKRAM VARMA 129](https://image.slidesharecdn.com/massspectrometry-191221153343/85/Mass-spectrometry-Analytical-Technique-129-320.jpg)