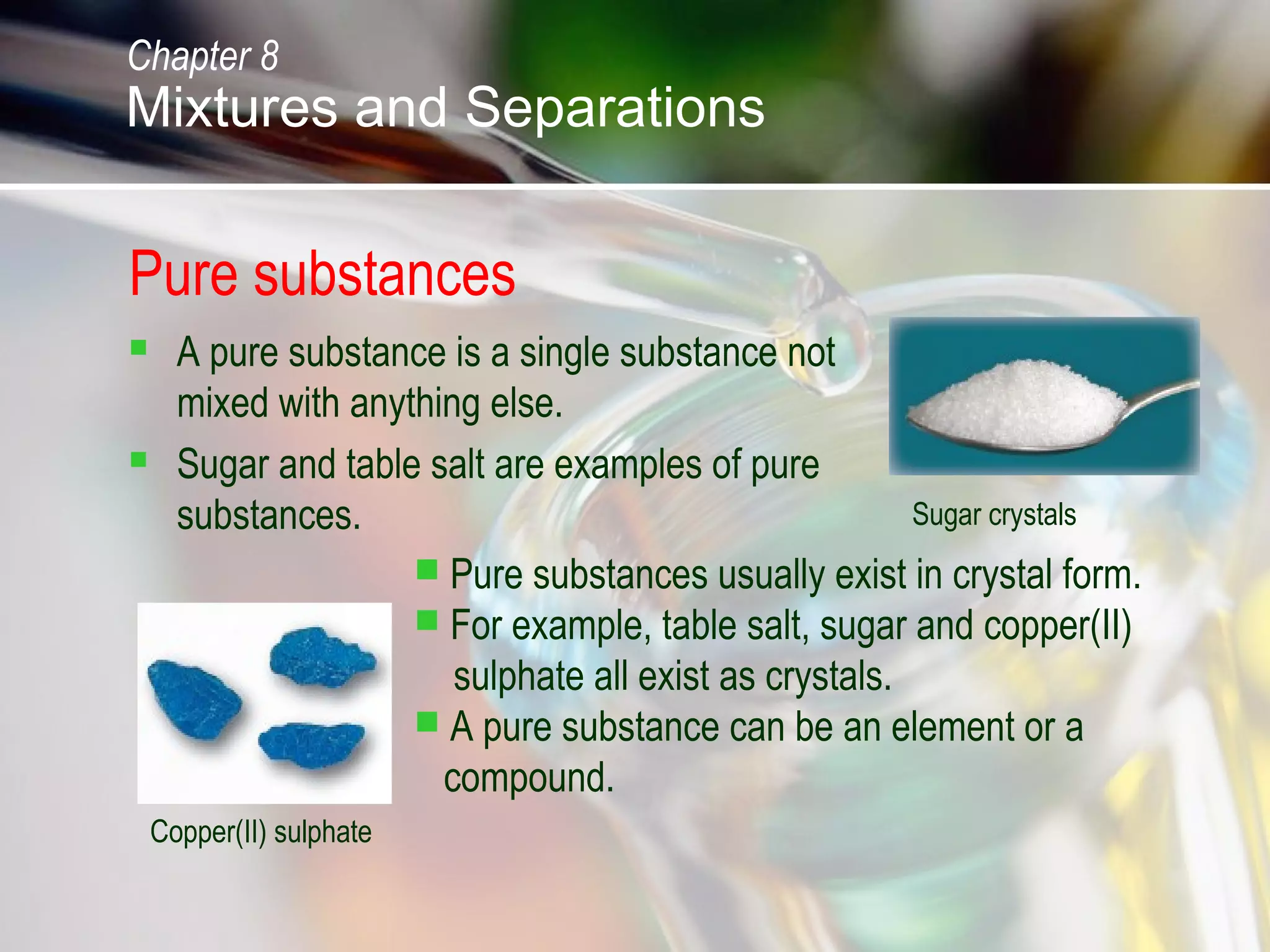
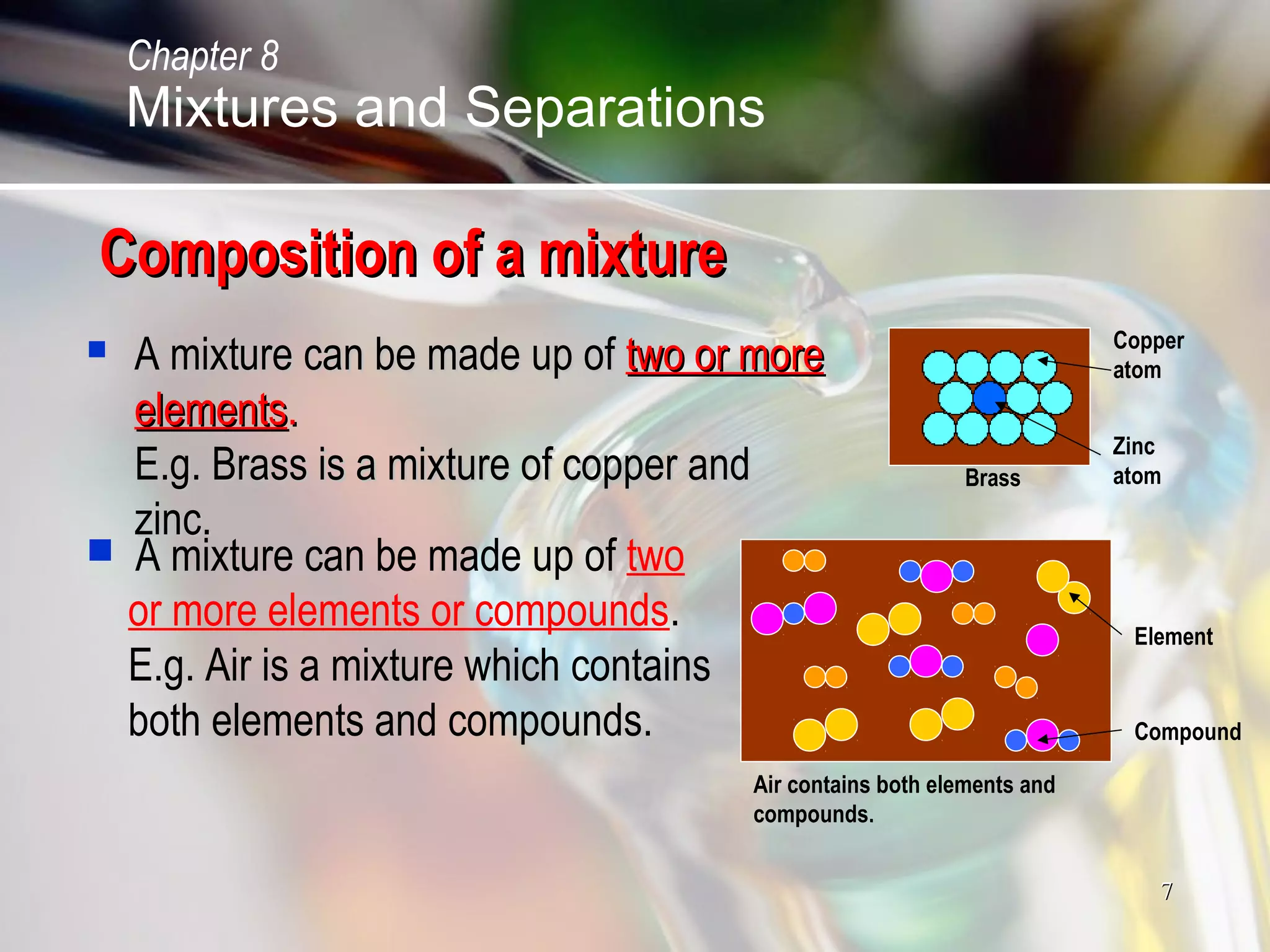
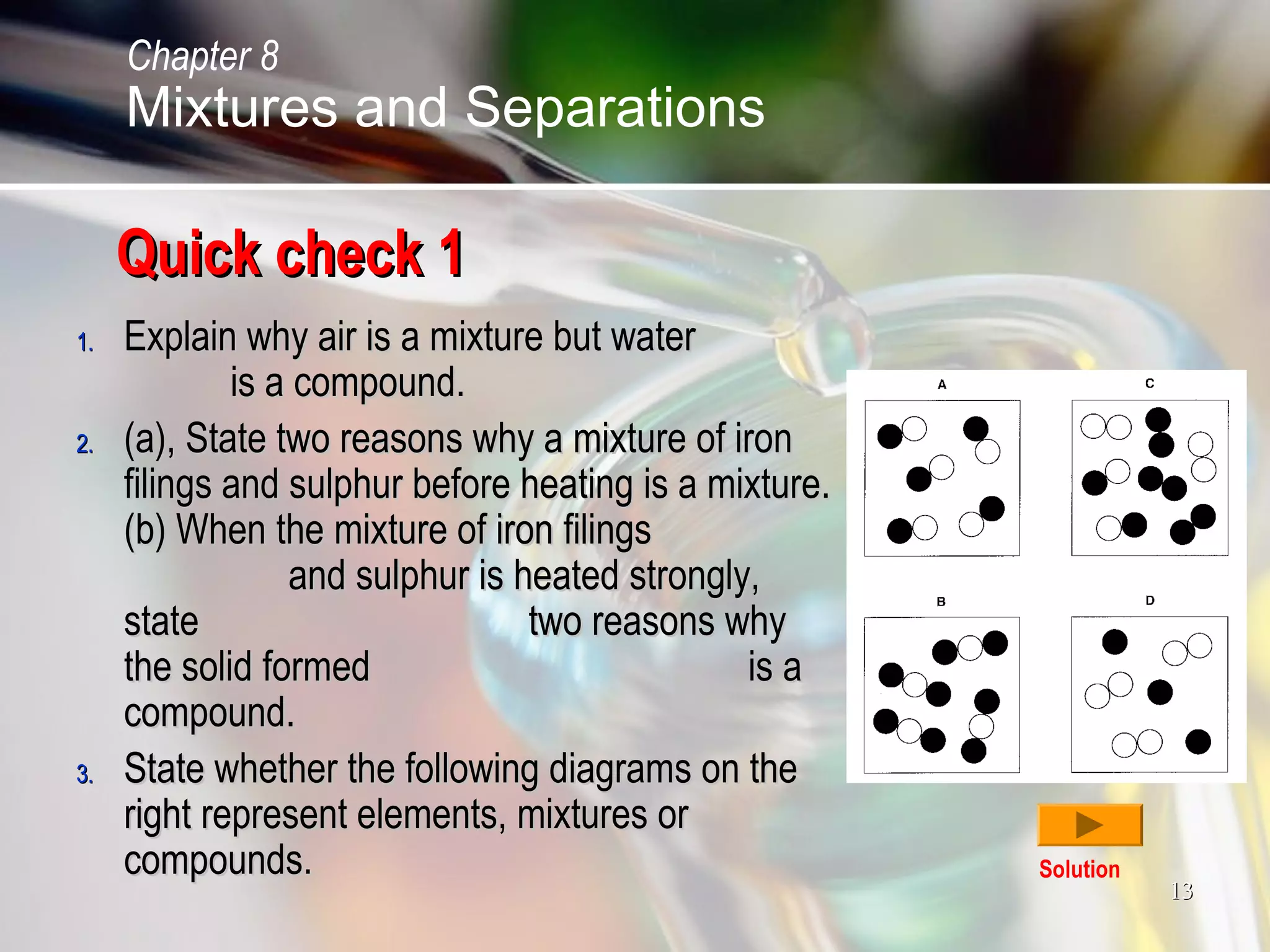
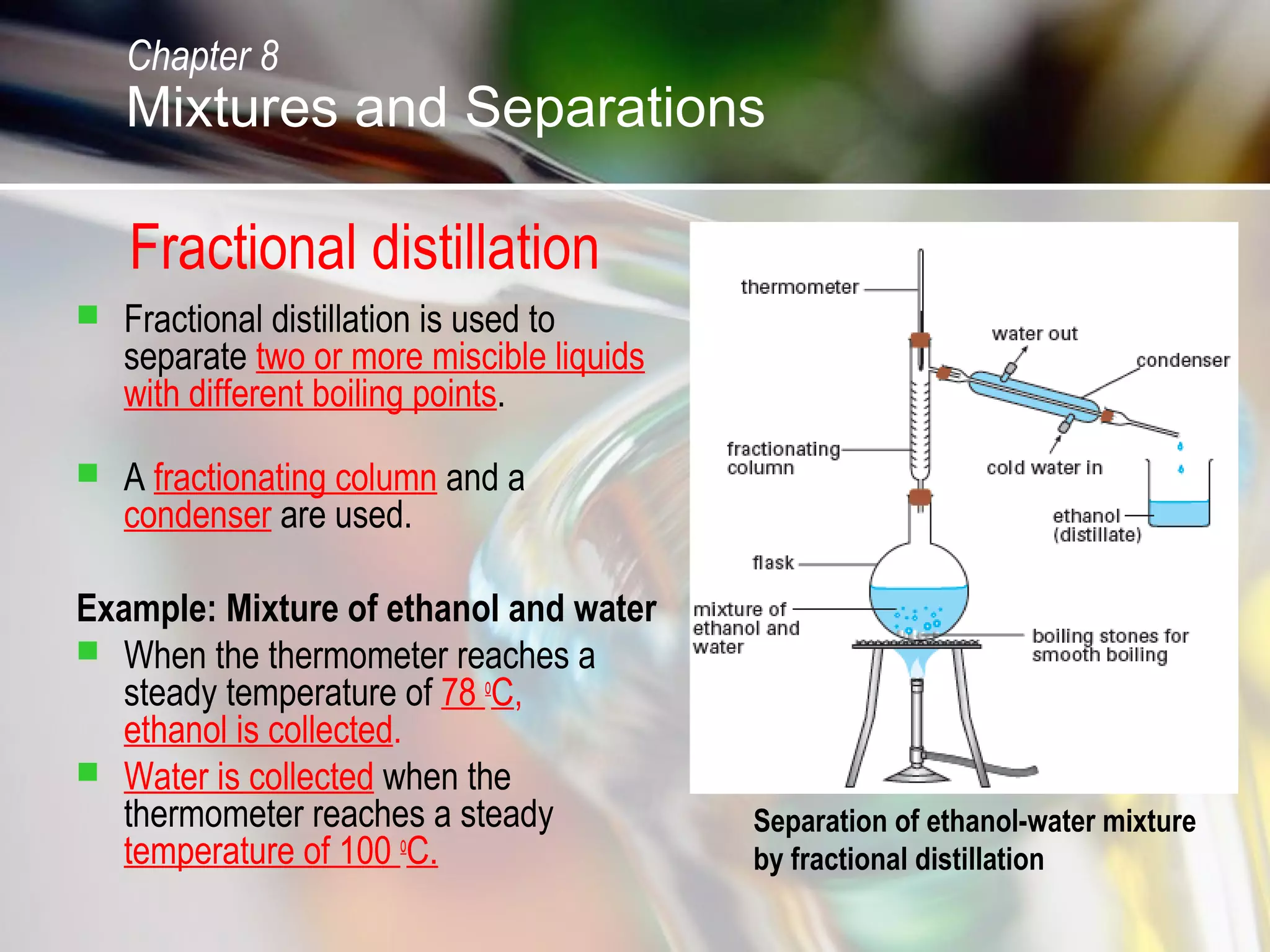
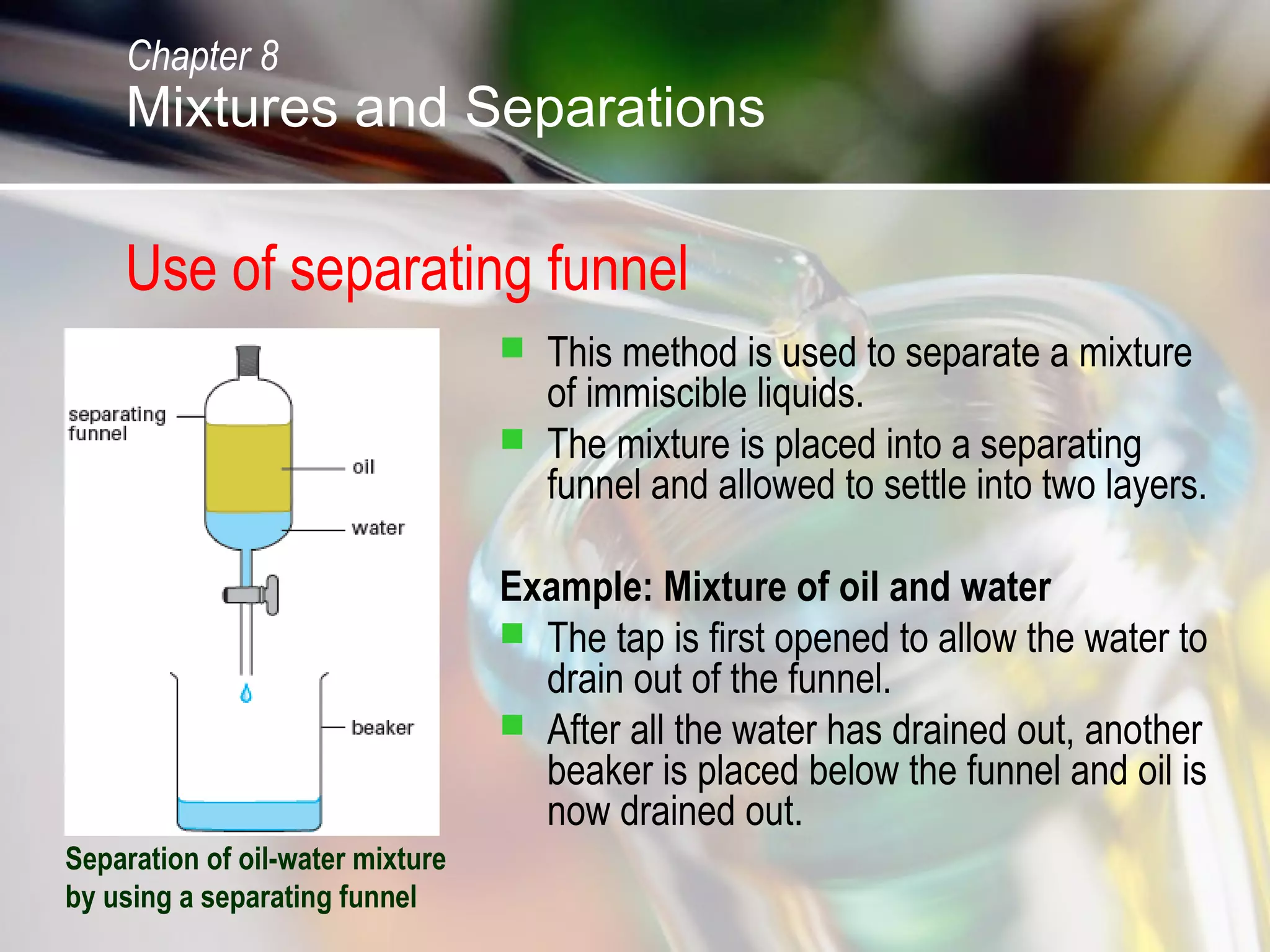
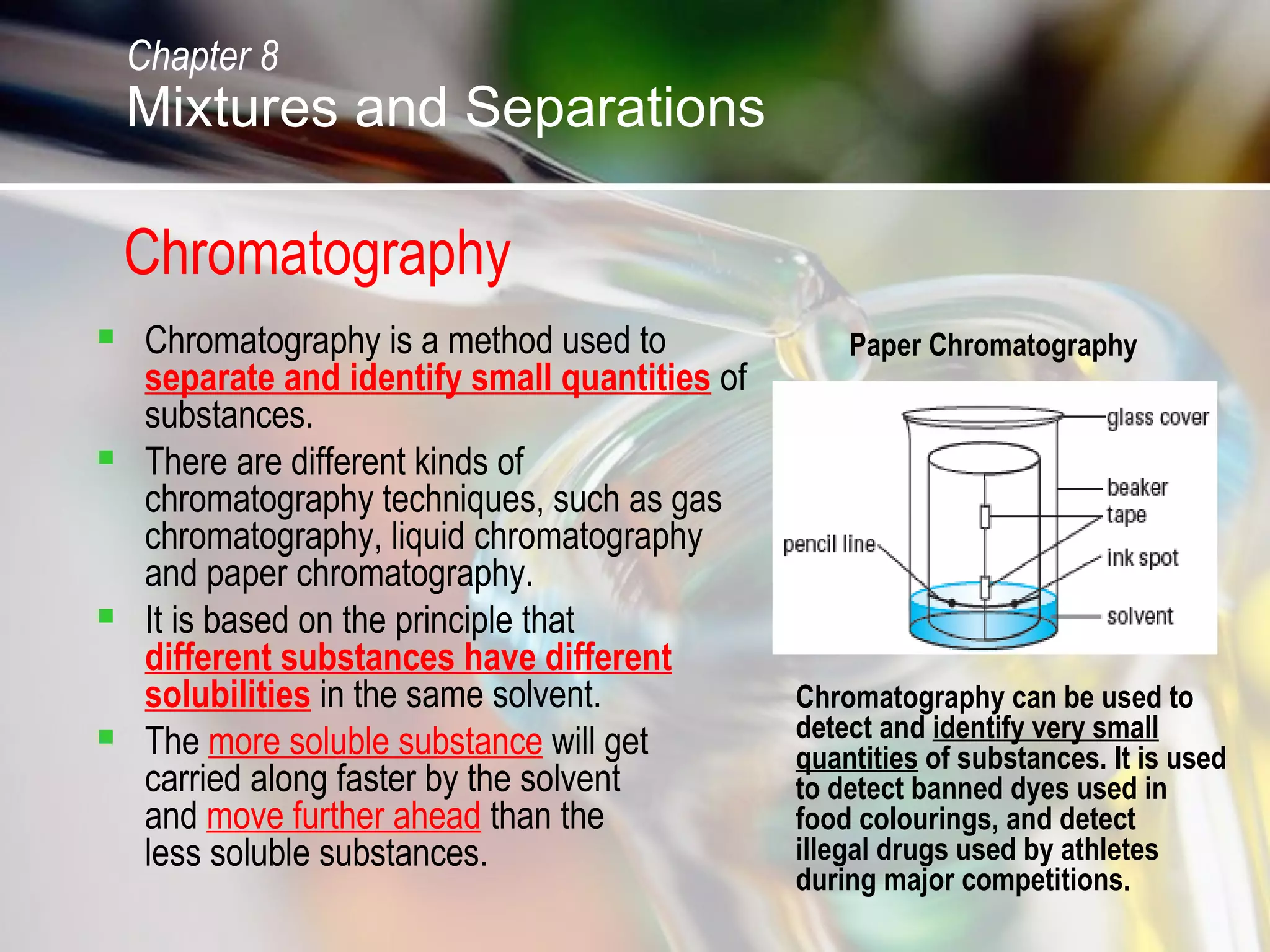
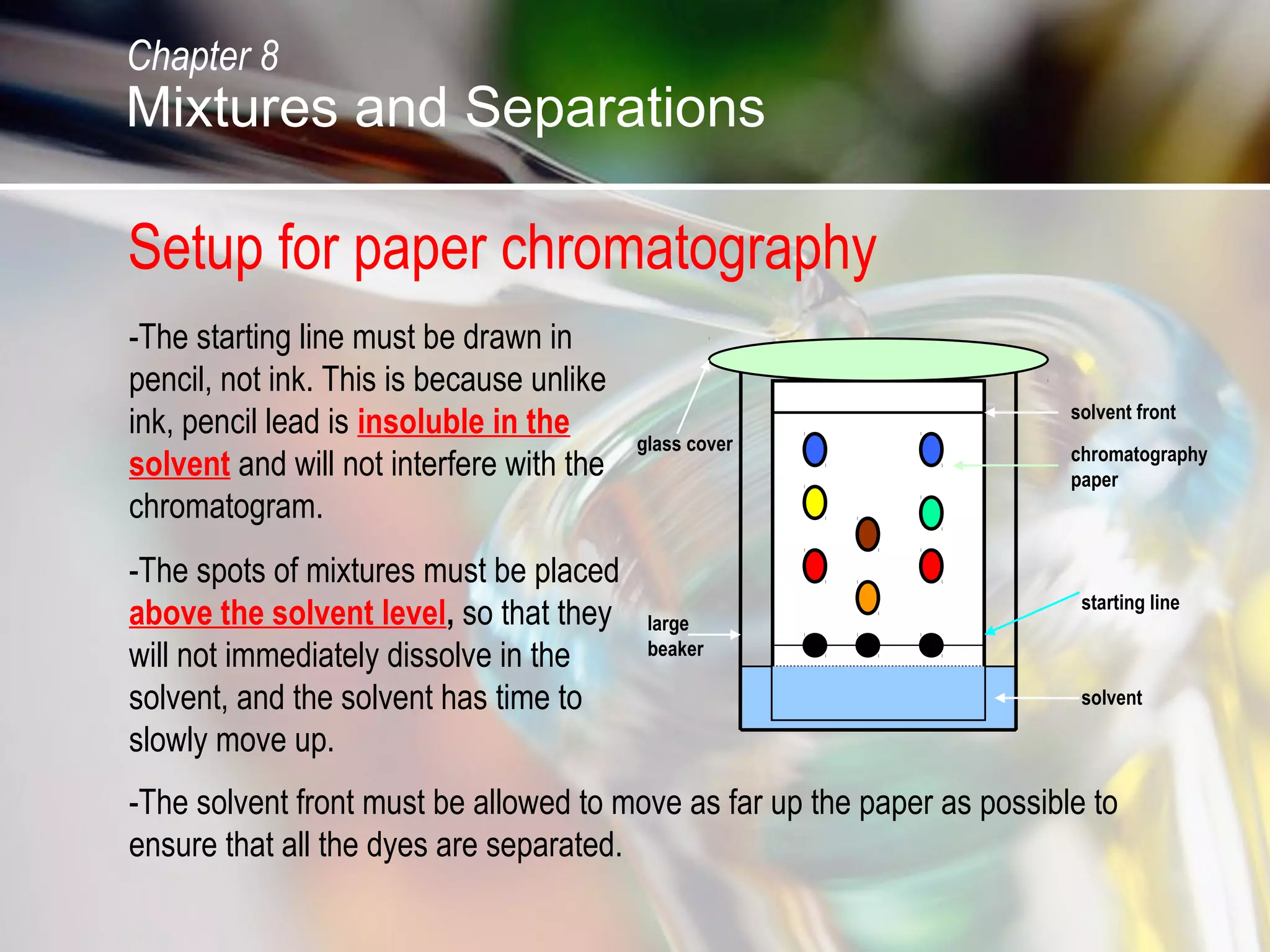
The document discusses various topics related to mixtures and separations:
- It identifies different types of solutions, suspensions, and colloids.
- It investigates how structure and temperature affect solubility of solids in water.
- It distinguishes among solutions, suspensions, and colloids and identifies suitable separation techniques based on differences in component properties of mixtures.
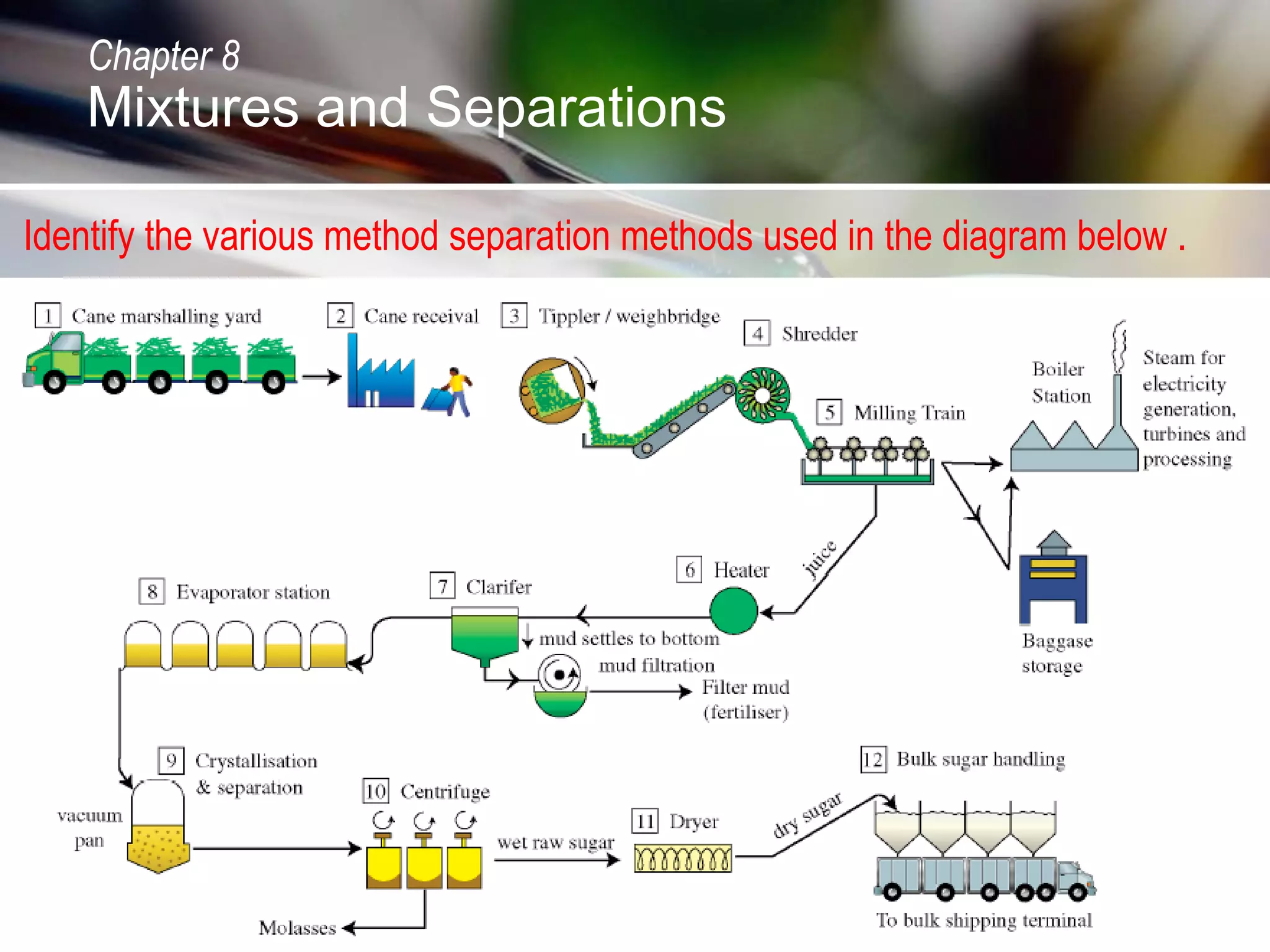
- It describes the extraction of sucrose from sugar cane.